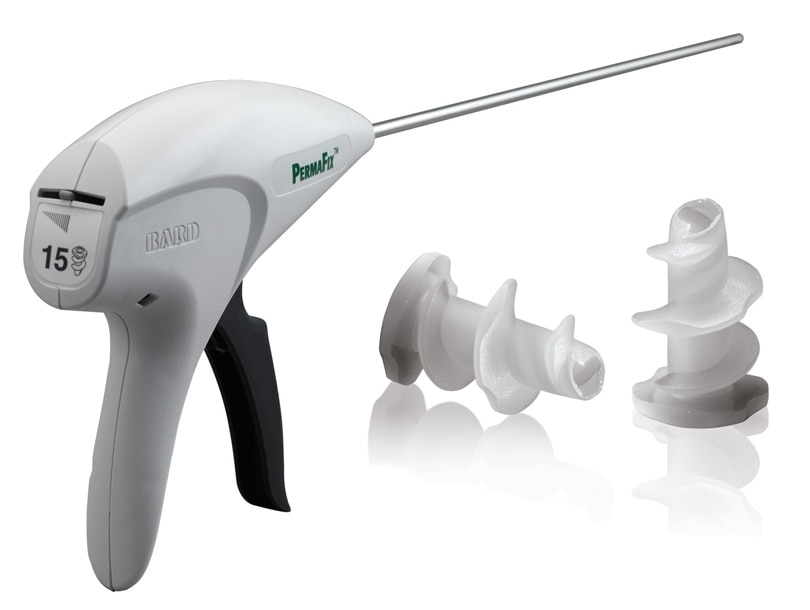
The unique fastener design of the PermaFix™ Permanent Fixation system means no sharp points are left behind in the patient. Preclinical data show that a hollow core allows tissue ingrowth through the fastener.1 The 5 mm depth of tissue purchase and consistent thread diameter from head to tip assure optimal tissue engagement during open and laparoscopic procedures. The PermaFix™ Permanent Fixation System has a 5 mm, low-profile delivery system that offers smooth, efficient deployment. Keeping track of thepre-loaded 15- or 30-fastener configurations is easy with an easy-to-see fastener gauge.
Canada (FR)
PermaFix™ Permanent Fixation System
Permanent acetal fasteners available in both a 15 and 30 count configuration delivered via a disposable 5 mm applier.

- Overview
- Products & Accessories
- EIFU & Resources
- FAQ
Preclinical testing suggests:
- Repair strength approximately 7x greater than maximum Intraabdominal Pressure (IAP)1, 2
- Threaded, hollow core allows for tissue in-growth through interior of fastener 2
- The consistent diameter of the threads from head to tip and fastener length are designed to maximize tissue engagement
- Obturator with piloting tip accurately pilots fastener through mesh and tissue
- Atraumatic blunt tip fastener with smooth head and no exposed points
- Innovative mechanical design enables a durable delivery system
- Molded polymer-based material similar to other implant devices
- Material not radiopaque
1. Twardowski ZJ et al “Intraabdominal Pressures during Natural Activities in Patients Treated with Continuous Ambulatory Peritoneal Dialysis” Nephron 44:129-135 1986. Cobb WS et al. "The Argument for Lightweight Polypropylene Mesh in Hernia Repair" Surgical Innovation. Vol 12 (1).63-69, 2005.
2. Data generated from a preclinical study using the SorbaFix™ Absorbable Fixation System and Composix™ L/P Mesh. Data on file. Results may not correlate to performance in humans.
INDICATIONS
The PermaFix™ Fixation System is indicated for the approximation of soft tissue and fixation of surgical mesh to tissues during open or laparoscopic surgical procedures, such as hernia repair.
CONTRAINDICATIONS
This device is not intended for use except as indicated.
Do not use this device where hemostasis cannot be verified visually after application. Contraindications associated with laparoscopic and open surgical procedures relative to mesh fixation apply, including but not limited to:
- Fixation of vascular or neural structures
- Fixation of bone and cartilage
This device should not be used in patients with a known allergy or hypersensitivity to acetal polymers.
Carefully inspect the area in the vicinity of the tissue being fastened to avoid inadvertent penetration of underlying structures such as nerves, vessels, viscera or bone. Use of the PermaFix™ Fixation System in the close vicinity of such underlying structures is contraindicated. For reference, the length of the fastener is 6.0 mm, the fastener head is another 0.7 mm (total 6.7 mm).
WARNINGS
The PermaFix™ Fixation System is intended for Single Use Only – DO NOT RESTERILIZE. Reuse, reprocessing, resterilization or repackaging may compromise the structural integrity and/or essential material and design characteristics that are critical to the overall performance of the device and may lead to device failure which may result in injury to the patient. Reuse, reprocessing, resterilization or repackaging may also create a risk of contamination of the device and/or cause patient infection or cross infection, including, but not limited to, the transmission of infectious diseases from one patient to another.
Contamination of the device may lead to injury, illness or death of the patient or end user.
If package is damaged or open, do not use product. Check package for damage prior to use.
Do not use beyond the expiration date on the package.
As with any implant the presence of bacterial contamination may enhance bacterial infection. Accepted surgical practice must be followed with respect to prevention of infection and drainage and closure of infected or contaminated wounds.
Users should be familiar with surgical procedures and techniques involving permanent fixation before employing PermaFix™ Fixation System fasteners for wound closure, as the risk of wound dehiscence may vary with the site of application and the material used. The device may not fixate through prosthetics from naturally derived material such as xenografts and allografts. Prosthetic should be evaluated for compatibility prior to use.
To prevent patient injury from the piloting tip, stay clear of vessels, nerves, bowel and viscera when entering the surgical site, manipulating tissue and fixating mesh. After use, the PermaFix™ Fixation System may be a potential biohazard. This device has a piloting tip, which should be considered a sharp even when the device is not actuated. Handle and dispose of in accordance with any local and federal laws regarding medical waste and sharps disposal requirements to prevent sharps injuries.
PRECAUTIONS
Please read all instructions before using the PermaFix™ Fixation System. Only persons having adequate medical training and familiarity with surgical techniques should perform surgical procedures. Consult the medical literature relative to technique, complications and hazards prior to any surgical procedure.
The PermaFix™ Fixation System can be used with most 5 mm trocars. Ensure compatibility by inserting the device into the trocar prior to introduction into the patient. The PermaFix™ Fixation System should enter and exit the trocar easily without excessive force. The use of too much force could damage the instrument.
Counter pressure should be applied on the target area. Avoid placing hand/finger directly over the area where fastener is being deployed to prevent injury.
Insertion of fasteners is possible into some collagenous structures such as ligaments and tendons, but the fasteners are not intended for use in bone or cartilage as this may damage the device.
Avoid excessive trigger force as this may damage the device. If the device locks, remove the device from the patient and lightly tap the trigger forward toward the tip to release.
If the device locks and cannot be separated from a fastener that has been deployed into tissue, you may rotate the device counter clockwise to free the device. The locked device should then be discarded and a new device should be used.
If the fastener does not deploy properly, remove the device from the patient and test the device in air to ensure proper fastener deployment. Once proper fastener deployment is confirmed, the device may be reinserted into the patient.
ADVERSE REACTIONS
Adverse reactions and potential complications associated with fixation devices such as the PermaFix™ Fixation System may include, but are not limited to the following: hemorrhage, pain, edema, and erythema at wound site; septicemia/infection; allergic reaction to acetal; hernia recurrence/wound dehiscence.
Please consult package insert for more detailed safety information and instructions for use.
BD-14699
1. Twardowski ZJ et al “Intraabdominal Pressures during Natural Activities in Patients Treated with Continuous Ambulatory Peritoneal Dialysis” Nephron 44:129-135 1986. Cobb WS et al. "The Argument for Lightweight Polypropylene Mesh in Hernia Repair" Surgical Innovation. Vol 12 (1).63-69, 2005.
2. Data generated from a preclinical study using the SorbaFix™ Absorbable Fixation System and Composix™ L/P Mesh. Data on file. Results may not correlate to performance in humans.
INDICATIONS
The PermaFix™ Fixation System is indicated for the approximation of soft tissue and fixation of surgical mesh to tissues during open or laparoscopic surgical procedures, such as hernia repair.
CONTRAINDICATIONS
This device is not intended for use except as indicated.
Do not use this device where hemostasis cannot be verified visually after application. Contraindications associated with laparoscopic and open surgical procedures relative to mesh fixation apply, including but not limited to:
- Fixation of vascular or neural structures
- Fixation of bone and cartilage
This device should not be used in patients with a known allergy or hypersensitivity to acetal polymers.
Carefully inspect the area in the vicinity of the tissue being fastened to avoid inadvertent penetration of underlying structures such as nerves, vessels, viscera or bone. Use of the PermaFix™ Fixation System in the close vicinity of such underlying structures is contraindicated. For reference, the length of the fastener is 6.0 mm, the fastener head is another 0.7 mm (total 6.7 mm).
WARNINGS
The PermaFix™ Fixation System is intended for Single Use Only – DO NOT RESTERILIZE. Reuse, reprocessing, resterilization or repackaging may compromise the structural integrity and/or essential material and design characteristics that are critical to the overall performance of the device and may lead to device failure which may result in injury to the patient. Reuse, reprocessing, resterilization or repackaging may also create a risk of contamination of the device and/or cause patient infection or cross infection, including, but not limited to, the transmission of infectious diseases from one patient to another.
Contamination of the device may lead to injury, illness or death of the patient or end user.
If package is damaged or open, do not use product. Check package for damage prior to use.
Do not use beyond the expiration date on the package.
As with any implant the presence of bacterial contamination may enhance bacterial infection. Accepted surgical practice must be followed with respect to prevention of infection and drainage and closure of infected or contaminated wounds.
Users should be familiar with surgical procedures and techniques involving permanent fixation before employing PermaFix™ Fixation System fasteners for wound closure, as the risk of wound dehiscence may vary with the site of application and the material used. The device may not fixate through prosthetics from naturally derived material such as xenografts and allografts. Prosthetic should be evaluated for compatibility prior to use.
To prevent patient injury from the piloting tip, stay clear of vessels, nerves, bowel and viscera when entering the surgical site, manipulating tissue and fixating mesh. After use, the PermaFix™ Fixation System may be a potential biohazard. This device has a piloting tip, which should be considered a sharp even when the device is not actuated. Handle and dispose of in accordance with any local and federal laws regarding medical waste and sharps disposal requirements to prevent sharps injuries.
PRECAUTIONS
Please read all instructions before using the PermaFix™ Fixation System. Only persons having adequate medical training and familiarity with surgical techniques should perform surgical procedures. Consult the medical literature relative to technique, complications and hazards prior to any surgical procedure.
The PermaFix™ Fixation System can be used with most 5 mm trocars. Ensure compatibility by inserting the device into the trocar prior to introduction into the patient. The PermaFix™ Fixation System should enter and exit the trocar easily without excessive force. The use of too much force could damage the instrument.
Counter pressure should be applied on the target area. Avoid placing hand/finger directly over the area where fastener is being deployed to prevent injury.
Insertion of fasteners is possible into some collagenous structures such as ligaments and tendons, but the fasteners are not intended for use in bone or cartilage as this may damage the device.
Avoid excessive trigger force as this may damage the device. If the device locks, remove the device from the patient and lightly tap the trigger forward toward the tip to release.
If the device locks and cannot be separated from a fastener that has been deployed into tissue, you may rotate the device counter clockwise to free the device. The locked device should then be discarded and a new device should be used.
If the fastener does not deploy properly, remove the device from the patient and test the device in air to ensure proper fastener deployment. Once proper fastener deployment is confirmed, the device may be reinserted into the patient.
ADVERSE REACTIONS
Adverse reactions and potential complications associated with fixation devices such as the PermaFix™ Fixation System may include, but are not limited to the following: hemorrhage, pain, edema, and erythema at wound site; septicemia/infection; allergic reaction to acetal; hernia recurrence/wound dehiscence.
Please consult package insert for more detailed safety information and instructions for use.
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
1. Twardowski ZJ et al “Intraabdominal Pressures during Natural Activities in Patients Treated with Continuous Ambulatory Peritoneal Dialysis” Nephron 44:129-135 1986. Cobb WS et al. "The Argument for Lightweight Polypropylene Mesh in Hernia Repair" Surgical Innovation. Vol 12 (1).63-69, 2005.
2. Data generated from a preclinical study using the SorbaFix™ Absorbable Fixation System and Composix™ L/P Mesh. Data on file. Results may not correlate to performance in humans.
INDICATIONS
The PermaFix™ Fixation System is indicated for the approximation of soft tissue and fixation of surgical mesh to tissues during open or laparoscopic surgical procedures, such as hernia repair.
CONTRAINDICATIONS
This device is not intended for use except as indicated.
Do not use this device where hemostasis cannot be verified visually after application. Contraindications associated with laparoscopic and open surgical procedures relative to mesh fixation apply, including but not limited to:
- Fixation of vascular or neural structures
- Fixation of bone and cartilage
This device should not be used in patients with a known allergy or hypersensitivity to acetal polymers.
Carefully inspect the area in the vicinity of the tissue being fastened to avoid inadvertent penetration of underlying structures such as nerves, vessels, viscera or bone. Use of the PermaFix™ Fixation System in the close vicinity of such underlying structures is contraindicated. For reference, the length of the fastener is 6.0 mm, the fastener head is another 0.7 mm (total 6.7 mm).
WARNINGS
The PermaFix™ Fixation System is intended for Single Use Only – DO NOT RESTERILIZE. Reuse, reprocessing, resterilization or repackaging may compromise the structural integrity and/or essential material and design characteristics that are critical to the overall performance of the device and may lead to device failure which may result in injury to the patient. Reuse, reprocessing, resterilization or repackaging may also create a risk of contamination of the device and/or cause patient infection or cross infection, including, but not limited to, the transmission of infectious diseases from one patient to another.
Contamination of the device may lead to injury, illness or death of the patient or end user.
If package is damaged or open, do not use product. Check package for damage prior to use.
Do not use beyond the expiration date on the package.
As with any implant the presence of bacterial contamination may enhance bacterial infection. Accepted surgical practice must be followed with respect to prevention of infection and drainage and closure of infected or contaminated wounds.
Users should be familiar with surgical procedures and techniques involving permanent fixation before employing PermaFix™ Fixation System fasteners for wound closure, as the risk of wound dehiscence may vary with the site of application and the material used. The device may not fixate through prosthetics from naturally derived material such as xenografts and allografts. Prosthetic should be evaluated for compatibility prior to use.
To prevent patient injury from the piloting tip, stay clear of vessels, nerves, bowel and viscera when entering the surgical site, manipulating tissue and fixating mesh. After use, the PermaFix™ Fixation System may be a potential biohazard. This device has a piloting tip, which should be considered a sharp even when the device is not actuated. Handle and dispose of in accordance with any local and federal laws regarding medical waste and sharps disposal requirements to prevent sharps injuries.
PRECAUTIONS
Please read all instructions before using the PermaFix™ Fixation System. Only persons having adequate medical training and familiarity with surgical techniques should perform surgical procedures. Consult the medical literature relative to technique, complications and hazards prior to any surgical procedure.
The PermaFix™ Fixation System can be used with most 5 mm trocars. Ensure compatibility by inserting the device into the trocar prior to introduction into the patient. The PermaFix™ Fixation System should enter and exit the trocar easily without excessive force. The use of too much force could damage the instrument.
Counter pressure should be applied on the target area. Avoid placing hand/finger directly over the area where fastener is being deployed to prevent injury.
Insertion of fasteners is possible into some collagenous structures such as ligaments and tendons, but the fasteners are not intended for use in bone or cartilage as this may damage the device.
Avoid excessive trigger force as this may damage the device. If the device locks, remove the device from the patient and lightly tap the trigger forward toward the tip to release.
If the device locks and cannot be separated from a fastener that has been deployed into tissue, you may rotate the device counter clockwise to free the device. The locked device should then be discarded and a new device should be used.
If the fastener does not deploy properly, remove the device from the patient and test the device in air to ensure proper fastener deployment. Once proper fastener deployment is confirmed, the device may be reinserted into the patient.
ADVERSE REACTIONS
Adverse reactions and potential complications associated with fixation devices such as the PermaFix™ Fixation System may include, but are not limited to the following: hemorrhage, pain, edema, and erythema at wound site; septicemia/infection; allergic reaction to acetal; hernia recurrence/wound dehiscence.
Please consult package insert for more detailed safety information and instructions for use.