Canada (FR)

- Overview
- Products & Accessories
- EIFU & Resources
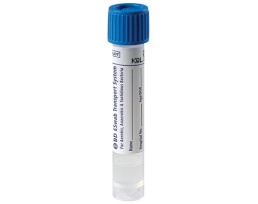
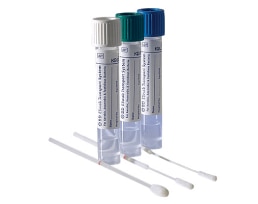
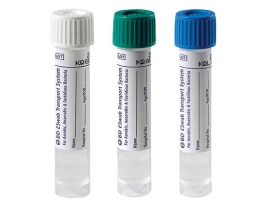
The BD ESwab™ collection and transport system helps collect clinical specimens containing aerobic, anaerobic and fastidious bacteria from the collection site, and transport them to the testing laboratory. In the lab, BD ESwab™ specimens are processed using standard clinical lab operating procedures for bacterial culture. The unique design of the flocked swab ensures optimal elution of the specimen into the transport medium.
The BD ESwab™ system is 1 mL of modified liquid Amies medium packaged with a nylon flocked swab.
The BD ESwab™ system provides 1 mL of sample suspension for Gram stain and multiple culture analyses.
Notes
* The system has been tested and validated in full compliance with CLSI standard M40-A: Quality Control of Microbiological Transport Systems, including for Neisseria gonorrhoeae survival at 24 hours.
ESwab is a registered trademark of COPAN ITALIA S.P.A ©2017
Notes
* The system has been tested and validated in full compliance with CLSI standard M40-A: Quality Control of Microbiological Transport Systems, including for Neisseria gonorrhoeae survival at 24 hours.
ESwab is a registered trademark of COPAN ITALIA S.P.A ©2017
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
Notes
* The system has been tested and validated in full compliance with CLSI standard M40-A: Quality Control of Microbiological Transport Systems, including for Neisseria gonorrhoeae survival at 24 hours.
ESwab is a registered trademark of COPAN ITALIA S.P.A ©2017