Canada (FR)
3DMax™ Light Mesh
Lighter-weight version of the popular 3DMax™ Mesh, featuring a large pore knit design.

- Overview
- Products & Accessories
- EIFU & Resources
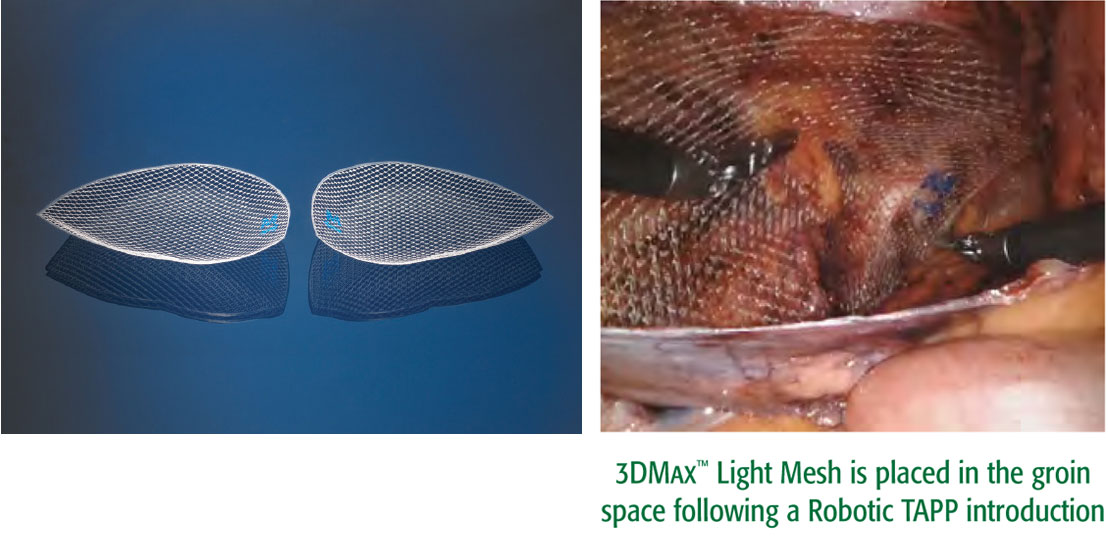
A lightweight 3D-shaped mesh for laparoscopic approaches such as TAPP, TEP and Robotic TAPP
This lighter-weight version of our popular 3DMax™ Mesh features a large pore knit. It is easy to deploy and provides excellent visibility. It is designed to conform to the inguinal anatomy and retains its shape following laparoscopic introduction, including a robotic approach.
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
Unique
- 3D shape developed by a laparoscopic surgeon.
- Designed to conform to the inguinal anatomy.
- Contour minimizes buckling that may be seen with flat mesh.
- Design may reduce the need for fixation.
Precise
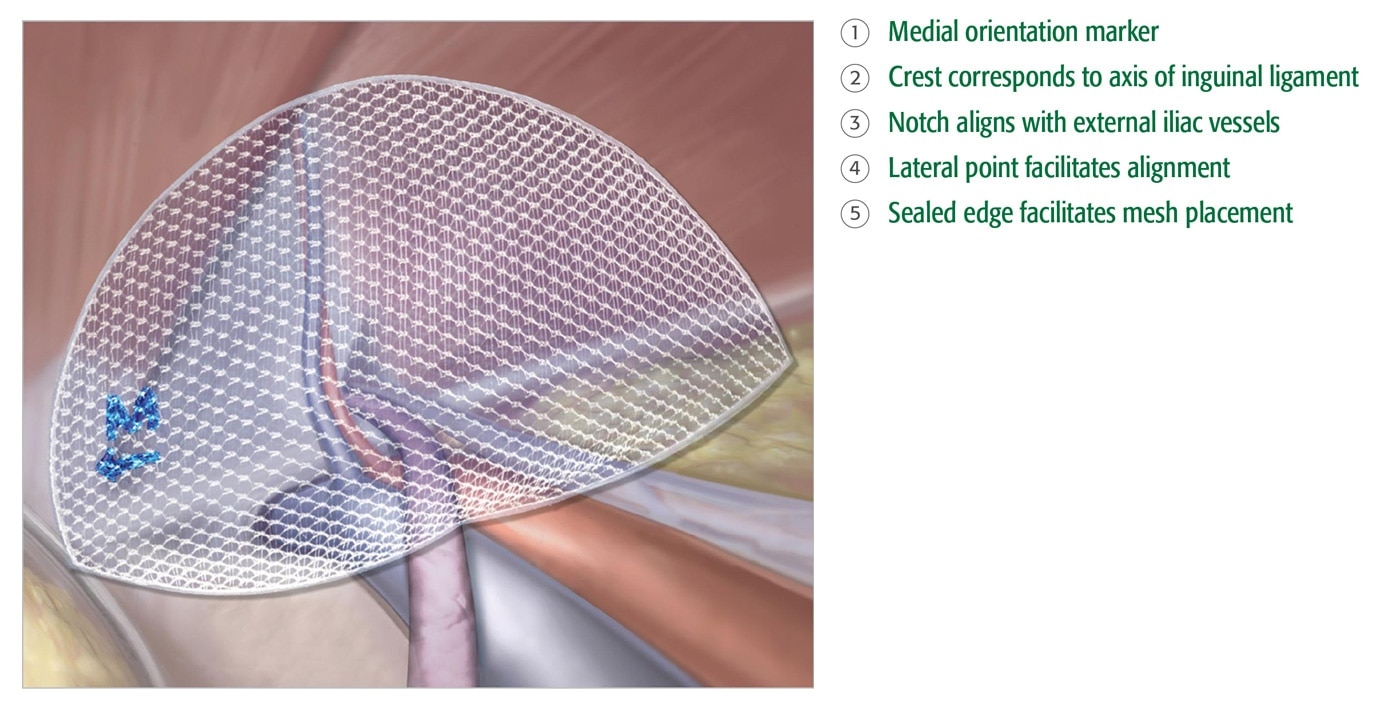
- Sealed edge and medial orientation marker facilitate accurate placement and positioning.
- Built-in memory maintains shape.
- 3DMax™ Light mesh is available in 3 sizes and in both left and right orientation.
Lighter Weight
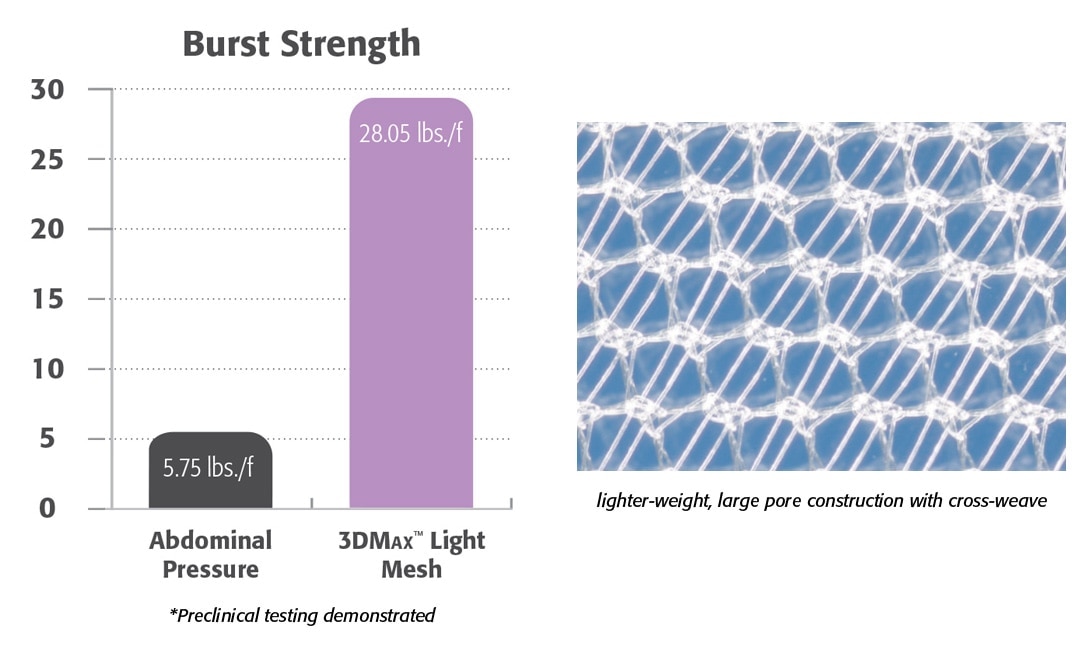
- Lighter-weight monofilament polypropylene mesh.
- Large pore knit provides excellent visibility.
- Preclinical data demonstrated the formation of a flexible and compliant abdominal wall.1
- TAPP
- TEP
- Robotic TAPP
1 Data on file. Results may not correlate to performance in humans.
Consult product labels and inserts for any indications, contraindications, hazards, warnings, cautions and instructions for use.
Indications
The 3DMax™ Light Mesh is indicated for use in the reinforcement of soft tissue where weakness exists, in the repair of inguinal hernias.
Contraindications
Literature reports that there may be a possibility for adhesion formation when polypropylene mesh is placed in direct contact with the bowel or viscera. Do not use polypropylene mesh in infants and children, whereby future growth will be compromised by use of such material.
Warnings
1. The use of any permanent mesh or patch in a contaminated or infected wound could lead to fistula formation and/or extrusion of the prosthesis.
2. If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require removal of the device.
Precautions
1. Do not cut or reshape the Bard 3DMax™ Light mesh as this may affect its effectiveness.
2. If sutures are used to secure the mesh in place, nonabsorbable monofilament sutures are recommended.
Adverse Reactions
Possible complications include seromas, adhesions, hematomas, inflammation, extrusion, fistula formation and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.
BD-14716
1 Data on file. Results may not correlate to performance in humans.
Consult product labels and inserts for any indications, contraindications, hazards, warnings, cautions and instructions for use.
Indications
The 3DMax™ Light Mesh is indicated for use in the reinforcement of soft tissue where weakness exists, in the repair of inguinal hernias.
Contraindications
Literature reports that there may be a possibility for adhesion formation when polypropylene mesh is placed in direct contact with the bowel or viscera. Do not use polypropylene mesh in infants and children, whereby future growth will be compromised by use of such material.
Warnings
1. The use of any permanent mesh or patch in a contaminated or infected wound could lead to fistula formation and/or extrusion of the prosthesis.
2. If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require removal of the device.
Precautions
1. Do not cut or reshape the Bard 3DMax™ Light mesh as this may affect its effectiveness.
2. If sutures are used to secure the mesh in place, nonabsorbable monofilament sutures are recommended.
Adverse Reactions
Possible complications include seromas, adhesions, hematomas, inflammation, extrusion, fistula formation and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.
BD-14716
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
1 Data on file. Results may not correlate to performance in humans.
Consult product labels and inserts for any indications, contraindications, hazards, warnings, cautions and instructions for use.
Indications
The 3DMax™ Light Mesh is indicated for use in the reinforcement of soft tissue where weakness exists, in the repair of inguinal hernias.
Contraindications
Literature reports that there may be a possibility for adhesion formation when polypropylene mesh is placed in direct contact with the bowel or viscera. Do not use polypropylene mesh in infants and children, whereby future growth will be compromised by use of such material.
Warnings
1. The use of any permanent mesh or patch in a contaminated or infected wound could lead to fistula formation and/or extrusion of the prosthesis.
2. If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require removal of the device.
Precautions
1. Do not cut or reshape the Bard 3DMax™ Light mesh as this may affect its effectiveness.
2. If sutures are used to secure the mesh in place, nonabsorbable monofilament sutures are recommended.
Adverse Reactions
Possible complications include seromas, adhesions, hematomas, inflammation, extrusion, fistula formation and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.
BD-14716


