1. Deeken CR, Matthews BD. “Characterization of the mechanical strength, resorption properties, and histologic characteristics of a fully absorbable material (Poly-4-hydroxybutyrate-Phasix™ Mesh) in a porcine model of hernia repair.” ISRN Surgery 2013; 1-12. RPT3807332.
2. DeMeester, Steven R, et al. Combination of surgical technique and bioresorbable mesh reinforcement of the crural repair leads to low early hernia recurrence rates with laparoscopic paraesophageal hernia repair. J Gastrointest Surg. 2020
Indications
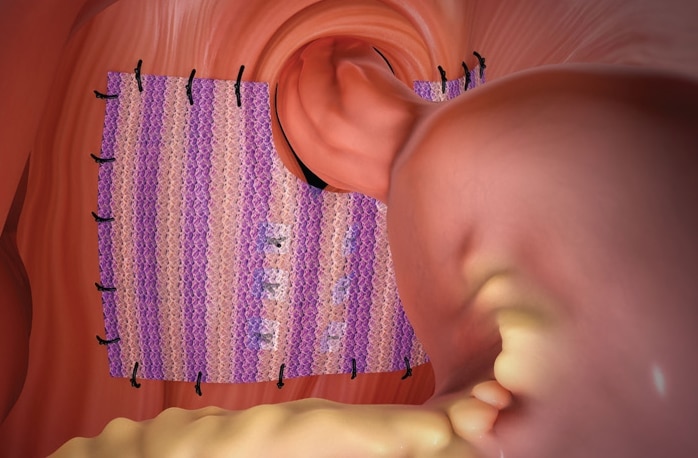
Phasix™ ST Mesh is indicated for use in the reinforcement of soft tissue, where weakness exists, in procedures involving soft tissue repair, such as for the repair of hernias, including hiatal hernias.
Contraindications
Because Phasix™ ST Mesh is fully resorbable, it should not be used in repairs where permanent wound or organ support from the mesh is required.
Warnings
Device manufacture involves exposure to tetracycline hydrochloride and kanamycin sulfate. The safety and product use for patients with hypersensitivities to these antibiotics is unknown. Use of this device in patients with known allergies to tetracycline hydrochloride or kanamycin sulfate should be avoided.
Ensure proper orientation; the coated side of the prosthesis should be oriented against the bowel or sensitive organs.
Do not place the uncoated mesh side against the bowel. There is a risk for adhesion formation or erosions when the uncoated mesh side is placed in direct contact with the bowel or viscera. (Reference Surface Orientation section.)
The safety and effectiveness of Phasix™ ST Mesh in bridging repairs has not been evaluated or established. The use of any synthetic mesh or patch in a contaminated or infected wound could lead to fistula formation and/or extrusion of the mesh and it is not recommended.
If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require removal of the mesh.
For hiatal hernia repair, the use of Phasix™ ST Mesh circumferentially around the esophagus is not recommended.
For hiatal hernia repair, the use of Phasix™ ST Mesh to bridge the hiatus is not recommended.
The safety and effectiveness of Phasix™ ST Mesh in the following applications has not been evaluated or established: Pregnant women, Pediatric use, Neural and Cardiovascular tissue.
Precautions
The safety and effectiveness of Phasix™ ST Mesh has not been evaluated in the presence of malignancies in the abdominopelvic cavity.
Adverse Reactions
In preclinical testing, Phasix™ ST Mesh elicited a minimal tissue reaction characteristic of foreign body response to a
substance. The tissue reaction resolved as the mesh was resorbed. Possible complications may include, but are not limited to, seroma, adhesion, hematoma, pain, infection, inflammation, allergic reaction, hemorrhage, extrusion, erosion, migration, fistula formation, and recurrence of the hernia or soft tissue defect. Possible complications in hiatal hernia repair may include esophageal erosion and dysphagia related to crural fibrosis.
Please consult package insert for more detailed safety information and instructions for use.