Sort by:
-
BD Surgiphor™ Antimicrobial Irrigation System
SKU/REF 910110
Ready-to-use and terminally sterile.
Every bottle. Every time.
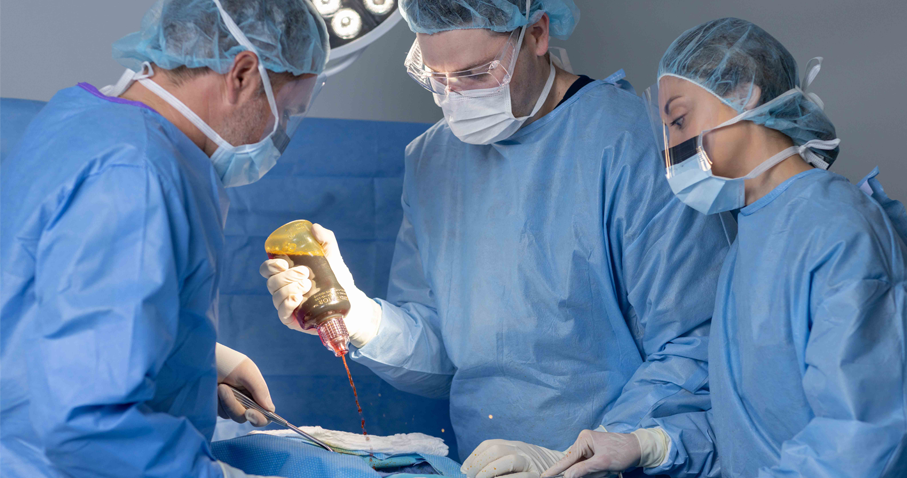

The Operating Room is a dynamic environment where every second matters. Your choice of wound irrigation can make a difference for surgeries. The new BD Surgiphor™ Antimicrobial Irrigation System is the only pre-mixed, terminally sterile dilute povidone-iodine (PVP-I) wound irrigation system. It is ready to use, enabling you to directly deliver a dilute PVP-I lavage to mechanically loosen and remove debris and foreign materials, including microorganisms from wounds during surgery. Using the BD Surgiphor™ System, you can reduce variability in wound irrigation processes, standardize wound irrigation practice and formulation for your patients, and align with current guidelines from the CDC1, WHO2, and the American Academy of Orthopaedic Surgeons (AAOS)3 to use dilute PVP-I for surgical wound irrigation.
Click on each hot spot to see how it can enable you to improve control, confidence and quality assurance in the OR.







AAOS: American Academy of Orthopaedic Surgeons. CDC: Centers for Disease Control and Prevention. USP: United States Pharmacopeial Convention. WHO: World Health Organization.
**PVP-I is an antimicrobial preservative contained within the bottled solution. The Surgiphor solution is not indicated for use as an antimicrobial at or within the wound site.
†Sterility assurance level in accordance with ISO 11137. *At initial time point of 7 days. Product should be used only within 24 hours of opening per IFU. ^Preservative solution with 0.5% PVP-I tested against Candida albicans (ATCC #10231), Pseudomonas aeruginosa (ATCC #9027), Aspergillus brasiliensis (ATCC #16404), Escherichia coli (ATCC #8739) and Staphylococcus aureus (ATCC #6538). **Against USP <51> organisms for up to 28 days.8 Once opened, the product should not be used on the patient after 24 hours. #Shown by the in-vitro antimicrobial efficacy of the PVP-I preservative in the BD Surgiphor™ solution.
Indication for use: Surgiphor™ Antimicrobial Irrigation System is intended to mechanically loosen and remove debris, and foreign materials, including microorganisms, from wounds. Contraindications: Surgiphor™ Antimicrobial Irrigation System should not be used in patients with known allergic reaction to any of the ingredients in the solutions. Surgiphor™ Antimicrobial Irrigation System should also not be combined with other irrigation or antiseptic solutions due to potential reactions and reduction in the effectiveness of the system. Not for use in neonates. Warnings: Do not use or mix with other cleansers, soaps, lotions, or ointments. Do not use for injection or infusion. Do not swallow. Do not use in eyes or ear canals. Discontinue use immediately if irritation or an allergic reaction occurs. Do not use if packaging is damaged or if seal integrity is compromised. Do not reuse Surgiphor™ solution after 24 hours. Precautions: Surgiphor™ solution may cause a temporary irritation and/or burning sensation on exposed skin in very rare cases. Surgiphor™ solution may cause allergic reactions such as rash or skin irritation in patients with iodine allergy. Anaphylaxis with the use of Surgiphor™ solution may occur in patients with severe iodine allergy. Federal law restricts this device to sale by or on the order of a licensed physician. Single patient use only. Not for at-home use. Please consult product insert for complete indications, contraindications, warnings, precautions, safety information and instructions for use.
References:

The BD Ambulatory Surgery Center portfolio of category-leading products, innovative solutions and dedicated support is designed to help ASCs improve clinical efficiency and enhance patient care.
We offer a full portfolio of surgical products
AAOS: American Academy of Orthopaedic Surgeons; CDC: Centers for Disease Control and Prevention; USP: United States Pharmacopeial Convention; WHO: World Health Organization
**PVP-I is an antimicrobial preservative contained within the bottled solution. The Surgiphor solution is not indicated for use as an antimicrobial at or within the wound site.
†Sterility assurance level in accordance with ISO 11137. *At initial time point of 7 days. Product should be used only within 24 hours of opening per IFU. ^Preservative solution with 0.5% PVP-I tested against Candida albicans (ATCC #10231), Pseudomonas aeruginosa (ATCC #9027), Aspergillus brasiliensis (ATCC #16404), Escherichia coli (ATCC #8739) and Staphylococcus aureus (ATCC #6538). **Against USP <51> organisms for up to 28 days.8 Once opened, the product should not be used on the patient after 24 hours. #Shown by the in-vitro antimicrobial efficacy of the PVP-I preservative in the BD Surgiphor™ solution.
Indication for use: Surgiphor™ Antimicrobial Irrigation System is intended to mechanically loosen and remove debris, and foreign materials, including microorganisms, from wounds. Contraindications: Surgiphor™ Antimicrobial Irrigation System should not be used in patients with known allergic reaction to any of the ingredients in the solutions. Surgiphor™ Antimicrobial Irrigation System should also not be combined with other irrigation or antiseptic solutions due to potential reactions and reduction in the effectiveness of the system. Not for use in neonates. Warnings: Do not use or mix with other cleansers, soaps, lotions, or ointments. Do not use for injection or infusion. Do not swallow. Do not use in eyes or ear canals. Discontinue use immediately if irritation or an allergic reaction occurs. Do not use if packaging is damaged or if seal integrity is compromised. Do not reuse Surgiphor™ solution after 24 hours. Precautions: Surgiphor™ solution may cause a temporary irritation and/or burning sensation on exposed skin in very rare cases. Surgiphor™ solution may cause allergic reactions such as rash or skin irritation in patients with iodine allergy. Anaphylaxis with the use of Surgiphor™ solution may occur in patients with severe iodine allergy. Federal law restricts this device to sale by or on the order of a licensed physician. Single patient use only. Not for at-home use. Please consult product insert for complete indications, contraindications, warnings, precautions, safety information and instructions for use.
References:
Our collection of literature on industries and on our offerings gives you information you can use to continue striving for excellence.
Training resources to help improve your clinical practices as part of our goal of advancing the world of health.
We support the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
We promote clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.
AAOS: American Academy of Orthopaedic Surgeons; CDC: Centers for Disease Control and Prevention; USP: United States Pharmacopeial Convention; WHO: World Health Organization
**PVP-I is an antimicrobial preservative contained within the bottled solution. The Surgiphor solution is not indicated for use as an antimicrobial at or within the wound site.
†Sterility assurance level in accordance with ISO 11137. *At initial time point of 7 days. Product should be used only within 24 hours of opening per IFU. ^Preservative solution with 0.5% PVP-I tested against Candida albicans (ATCC #10231), Pseudomonas aeruginosa (ATCC #9027), Aspergillus brasiliensis (ATCC #16404), Escherichia coli (ATCC #8739) and Staphylococcus aureus (ATCC #6538). **Against USP <51> organisms for up to 28 days.8 Once opened, the product should not be used on the patient after 24 hours. #Shown by the in-vitro antimicrobial efficacy of the PVP-I preservative in the BD Surgiphor™ solution.
Indication for use: Surgiphor™ Antimicrobial Irrigation System is intended to mechanically loosen and remove debris, and foreign materials, including microorganisms, from wounds. Contraindications: Surgiphor™ Antimicrobial Irrigation System should not be used in patients with known allergic reaction to any of the ingredients in the solutions. Surgiphor™ Antimicrobial Irrigation System should also not be combined with other irrigation or antiseptic solutions due to potential reactions and reduction in the effectiveness of the system. Not for use in neonates. Warnings: Do not use or mix with other cleansers, soaps, lotions, or ointments. Do not use for injection or infusion. Do not swallow. Do not use in eyes or ear canals. Discontinue use immediately if irritation or an allergic reaction occurs. Do not use if packaging is damaged or if seal integrity is compromised. Do not reuse Surgiphor™ solution after 24 hours. Precautions: Surgiphor™ solution may cause a temporary irritation and/or burning sensation on exposed skin in very rare cases. Surgiphor™ solution may cause allergic reactions such as rash or skin irritation in patients with iodine allergy. Anaphylaxis with the use of Surgiphor™ solution may occur in patients with severe iodine allergy. Federal law restricts this device to sale by or on the order of a licensed physician. Single patient use only. Not for at-home use. Please consult product insert for complete indications, contraindications, warnings, precautions, safety information and instructions for use.
References: