We’re doubling down on what we do best—accessing our vast experience to help solve the healthcare community’s biggest challenges, working with urgency, as we innovate and ensure our front lines have the innovative solutions and tools they need to address this pandemic and enhance patient care, as they impact lives around the world.
COVID-19 Vaccination Preparedness
Delivering a path out of the crisis together

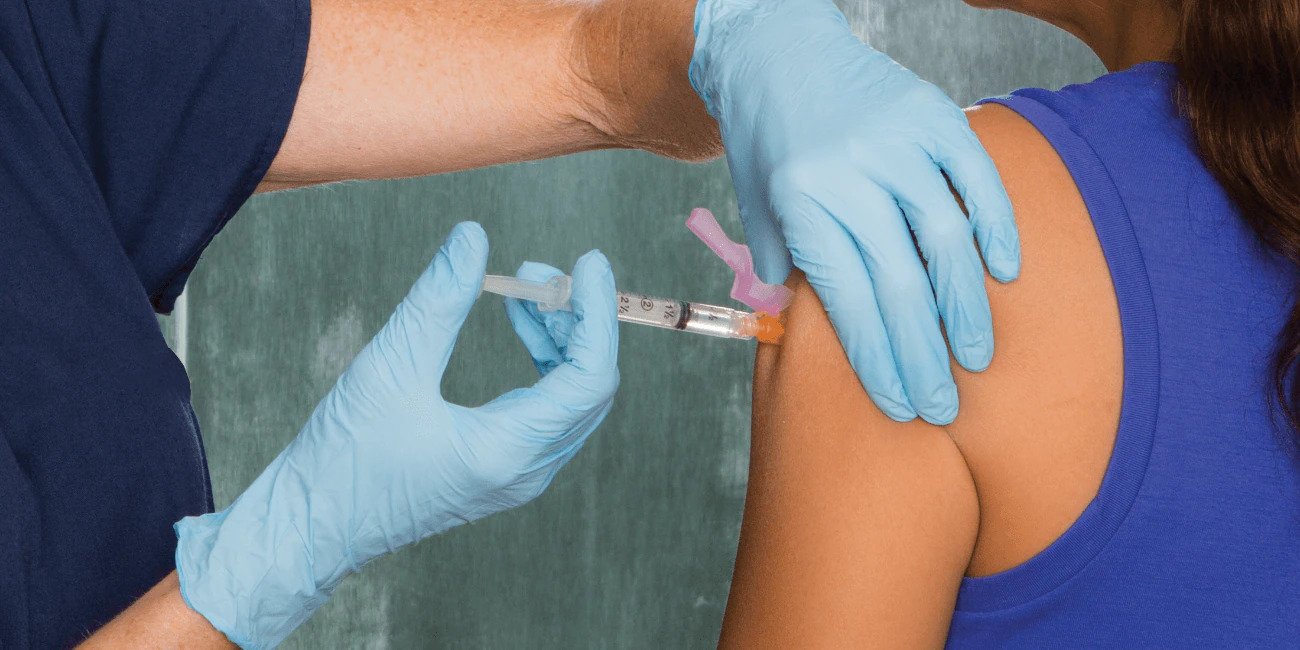
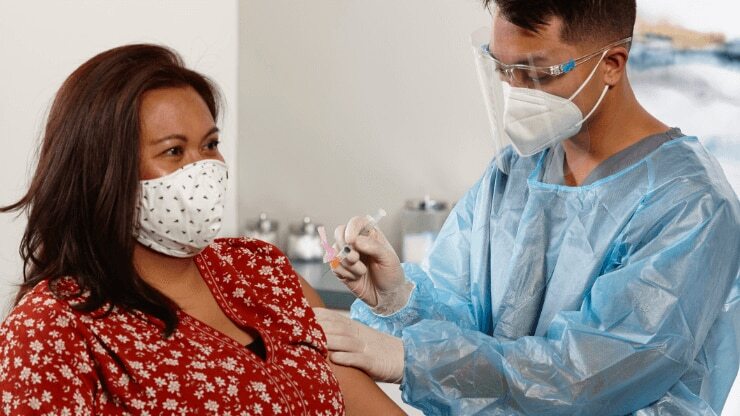
BD has mobilized and our devices are set to deliver the COVID-19 vaccine to the world
In the midst of this global pandemic, we understand the responsibility we carry as the world’s largest manufacturer of injection devices. This position of leadership is why we responded to the crisis early—rapidly scaling up our manufacturing operations. We’re here now, delivering a path out of this crisis, so we can enable healthcare providers to deliver the best possible patient care, which includes meeting the critical need for vaccinations.
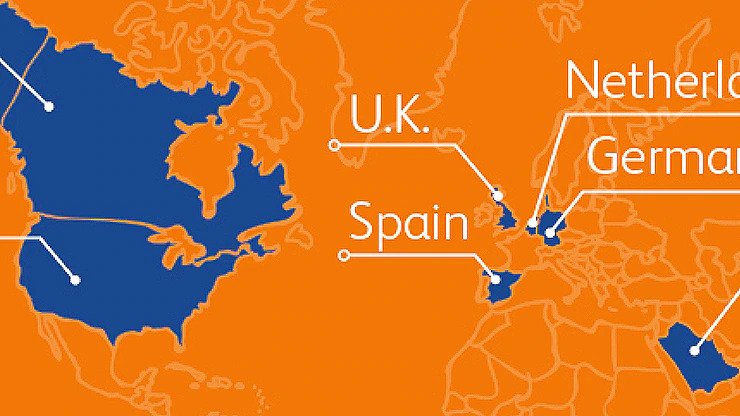
BD has already committed 2 billion injection devices to countries and nongovernmental organizations around the world*
* Publicly confirmed as of June 2021
The journey of a vaccine delivery device
BD has been by the side of vaccine developers and healthcare providers for decades, ensuring the availability and safe administration of vaccines from polio to the seasonal flu.
BD is partnering with governments and NGOs to ensure we deliver the right type of injection device in the right quantities to the right place at the right time.
BD has accelerated production of needles and syringes to ensure continuity of supply to our customers, while prioritizing patient care and meeting the requests for heightened vaccination needs.
** This product has not been FDA cleared or approved; but has been authorized by FDA under an EUA for use by authorized laboratories.
This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens.
This product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
BD-22503 (06/21)