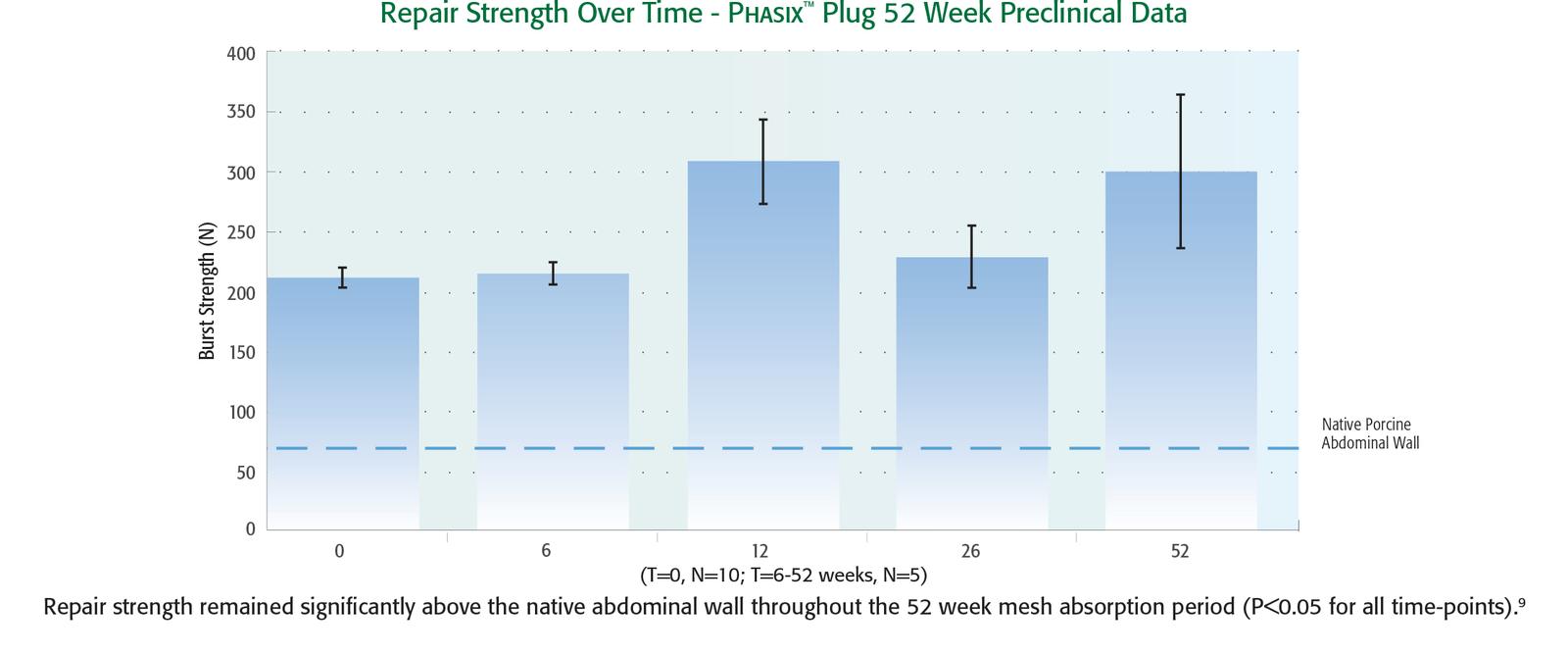
1. Millikan KW., Doolas A. “A Long-Term Evaluation of the Modified Mesh-Plug Hernioplasty in Over 2,000 Patients.” Hernia. 2008 Jun; 12(3): 257-260.
2. Phasix™ Plug and Patch Instructions for Use
3. Martin DP, Williams SF. “Medical Applications of Poly-4-Hydroxybutyrate: A Strong Flexible Absorbable Biomaterial.”
Biochemical Engineering Journal. 2003 (16): 97-105.
4. Benchtop test data on file at C. R. Bard, Inc. Results may not correlate to performance in humans.
5. Preclinical data on file at C. R. Bard, Inc. Results may not correlate to performance in humans.
6. Nienhuijs S et. al.“Chronic pain after mesh repair of inguinal hernia: a systematic review”. Am J Surg. 2007;194(3):394-400.
7. Demierer S, et. al. “The Effect of Polypropylene Mesh on Ilioinguinal Nerve in Open Mesh Repair of Groin Hernia.” Journal of Surgical Research 2006 (131): 175-181.
Indications for Use
The Phasix™ Plug and Patch is indicated for reinforcement of soft tissue where weakness exists, in procedures involving soft tissue repair, such as groin hernia defects.
Contraindications
Because Phasix™ Plug and Patch is fully resorbable, it should not be used in repairs where permanent wound or organ support from the mesh is required.
Warnings
Placement of Phasix™ Plug and Patch in direct contact with bowel or viscera is not recommended. The safety and product use for patients with hypersensitivities to tetracycline hydrochloride or kanamycin sulfate is unknown. Use of this device in patients with known allergies to these antibiotics should be avoided. The safety and effectiveness of Phasix™ Plug and Patch in pediatric use and use in pregnant women has not been established. If an infection develops, treat the infection aggressively. An unresolved infection may require removal of the device.
Adverse Reactions
Possible complications include infection, seroma, pain, mesh migration, wound dehiscence, hemorrhage, adhesions, hematoma, inflammation, extrusion and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use
.