BD-16948
Ventrio™ Hernia Patch
Self-expanding polypropylene and ePTFE patch for soft tissue reconstruction with SorbaFlex™ Memory Technology.

- Overview
- EIFU & Resources
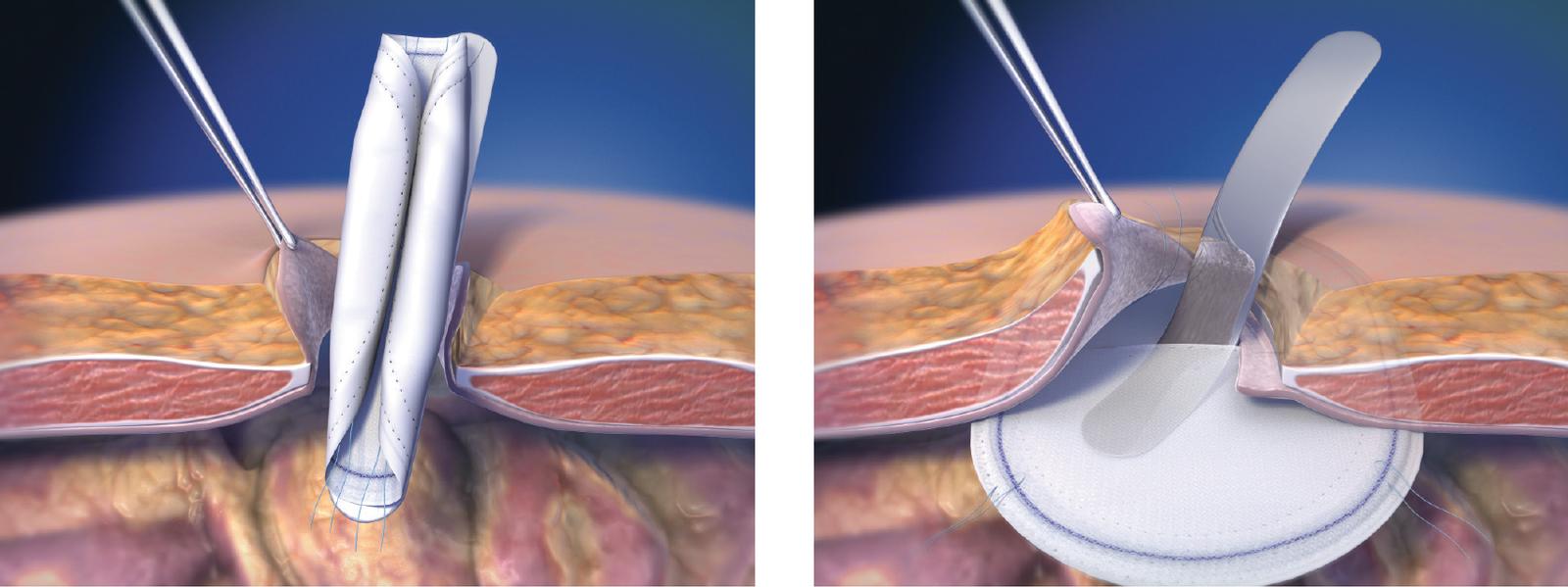
- Springs open, lays flat and maintains shape.
- Facilitates easy placement and positioning throughout the ventral repair.
- Absorption of the PDO monofilament occurs in vivo by means of hydrolysis and is essentially complete in 6–8 months, leaving less implant material behind.1
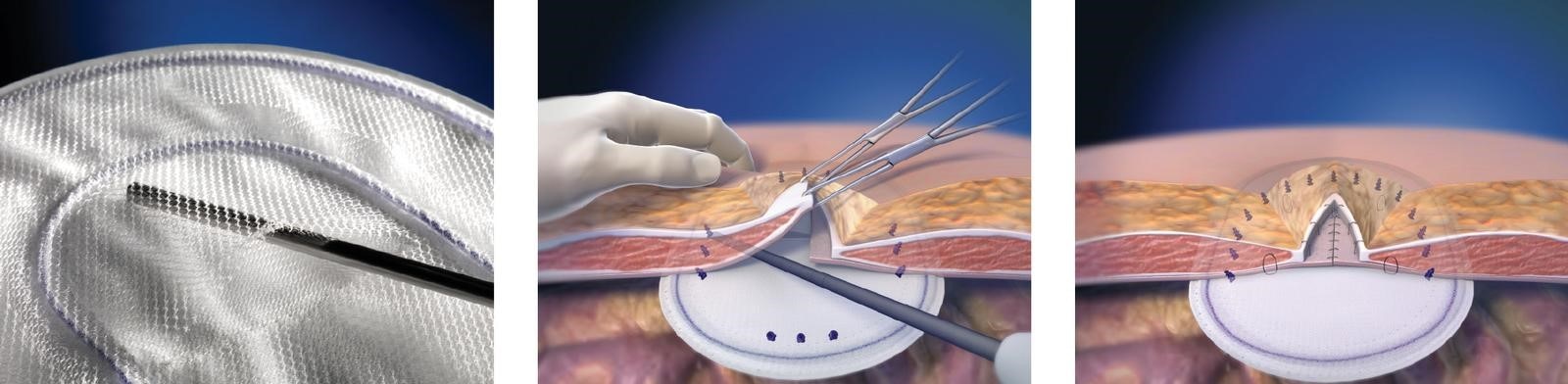
- Easy handling and positioning.
- Allows the use of mechanical fixation for increased efficiency.
- Monofilament polypropylene mesh provides fast tissue ingrowth and incorporation, eliminating the need for permanent transfixation sutures.
- Submicronic ePTFE side minimizes tissue attachment to the patch.
- ePTFE is complemented by monofilament polypropylene and polydioxanone.
- Used in general surgery for many years with success demonstrated by clinical outcomes.
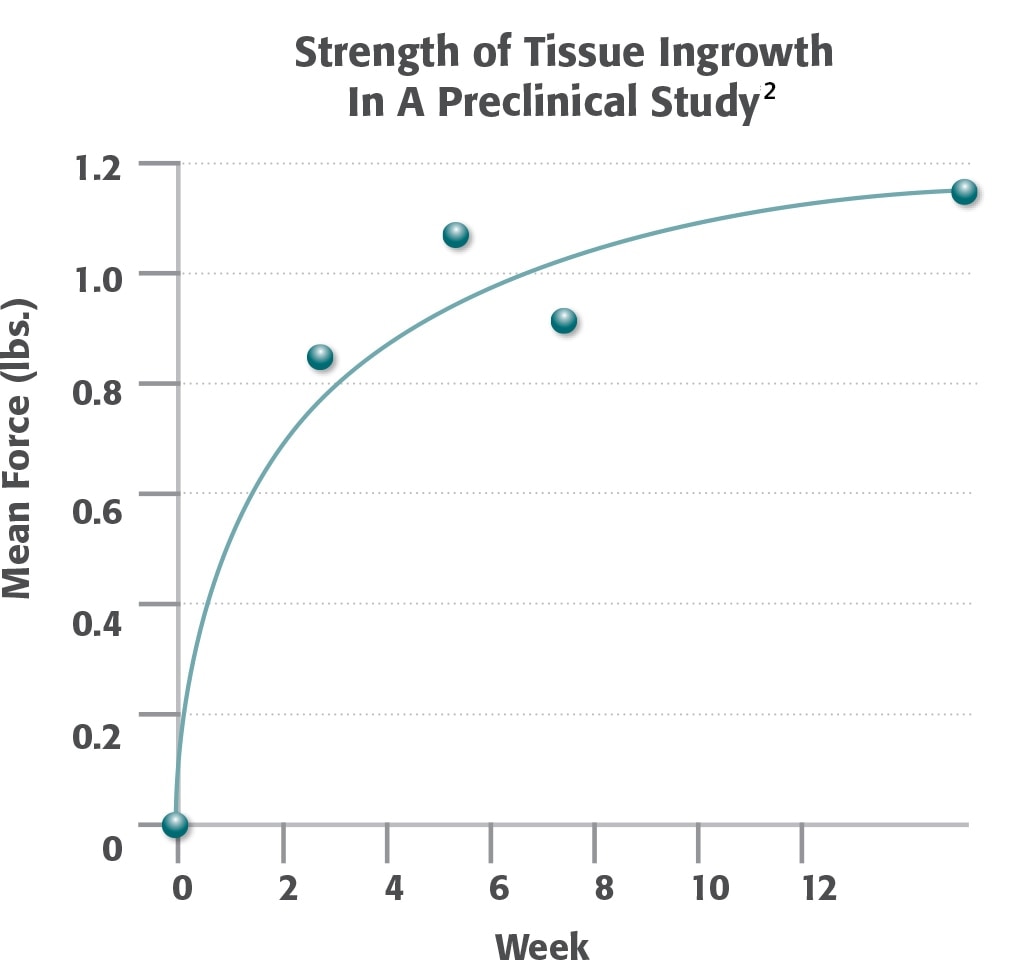
Logarithmic regression curve of mean force of lap-shear strength as a function of time. 74% of the 12 week strength is achieved by 2 weeks post-operatively.

1 Data generated in preclinical model. Data may not correlate to performance in humans.
2 Majercik, S. et al. “Strength in tissue attachment to mesh after ventral hernia repair with synthetic composite mesh in a porcine model.” Surg Endosc (2006) 20: 1671-1674.
INDICATIONS
The Ventrio™ Hernia Patch is indicated for use in the reconstruction of soft tissue deficiencies, such as for the repair of hernias.
CONTRADICTIONS
Literature reports that there may be a possibility for adhesion formation when the polypropylene is placed in contact with the bowel or viscera.
WARNINGS
1. Do not cut or reshape the Ventrio™ Hernia Patch, as this could affect its effectiveness. Care should be taken not to cut or nick the SorbaFlex™ PDO monofilament. If the SorbaFlex™ PDO monofilament is cut or damaged during insertion or fixation, additional complications may include bowel or skin perforation and infection.
2. Follow proper folding techniques for all patches as described in the Instructions for Use as other folding techniques may compromise the SorbaFlex™ PDO monofilament.
3. Ensure proper orientation; the solid white surface (ePTFE) must be oriented against the bowel or sensitive organs. Do not place the mesh surface against the bowel. There may be a possibility for adhesion formation when mesh is placed in direct contact with the bowel or viscera.
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation, infection, allergic reaction, and recurrence of the hernia or soft tissue defect. If theSorbaFlex™ PDO monofilament is cut or damaged during insertion or fixation, additional complications may include bowel or skin perforation and infection.
Please consult package insert for more detailed safety information and instructions for use.
BD-16948
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
1 Data generated in preclinical model. Data may not correlate to performance in humans.
2 Majercik, S. et al. “Strength in tissue attachment to mesh after ventral hernia repair with synthetic composite mesh in a porcine model.” Surg Endosc (2006) 20: 1671-1674.
INDICATIONS
The Ventrio™ Hernia Patch is indicated for use in the reconstruction of soft tissue deficiencies, such as for the repair of hernias.
CONTRADICTIONS
Literature reports that there may be a possibility for adhesion formation when the polypropylene is placed in contact with the bowel or viscera.
WARNINGS
1. Do not cut or reshape the Ventrio™ Hernia Patch, as this could affect its effectiveness. Care should be taken not to cut or nick the SorbaFlex™ PDO monofilament. If the SorbaFlex™ PDO monofilament is cut or damaged during insertion or fixation, additional complications may include bowel or skin perforation and infection.
2. Follow proper folding techniques for all patches as described in the Instructions for Use as other folding techniques may compromise the SorbaFlex™ PDO monofilament.
3. Ensure proper orientation; the solid white surface (ePTFE) must be oriented against the bowel or sensitive organs. Do not place the mesh surface against the bowel. There may be a possibility for adhesion formation when mesh is placed in direct contact with the bowel or viscera.
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation, infection, allergic reaction, and recurrence of the hernia or soft tissue defect. If theSorbaFlex™ PDO monofilament is cut or damaged during insertion or fixation, additional complications may include bowel or skin perforation and infection.
Please consult package insert for more detailed safety information and instructions for use.
BD-16948

