BD-14803
Ventralex™ Hernia Patch
A clinically proven umbilical hernia repair solution designed for ventral, incisional, umbilical and epigastric hernia repair as well as trocar site closure.


- Overview
- Products & Accessories
- EIFU & Resources
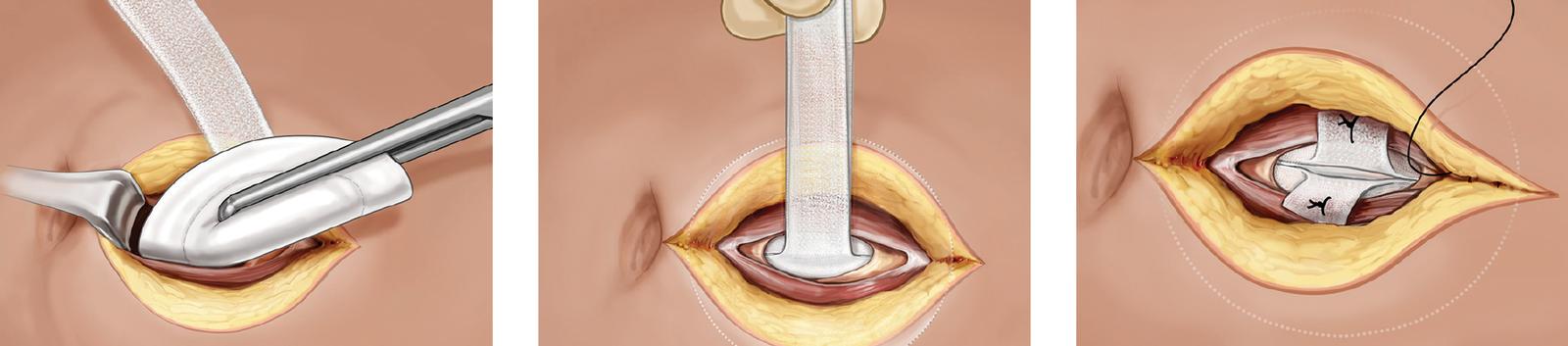
Technique and Placement
- Simple deployment technique
- Tension-free abdominal wall repair
- Minimum fixation required
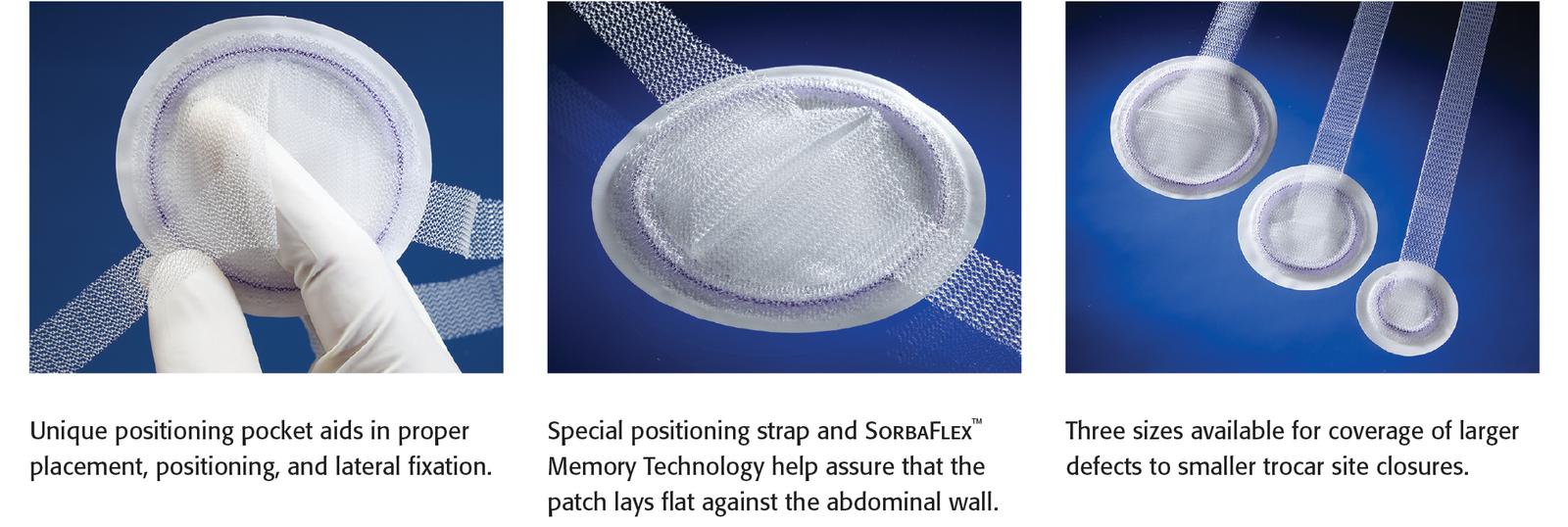
Positioning
- Pocket and strap help facilitate placement, positioning and fixation
- SorbaFlex™ Memory technology allows the patch to “spring open,” lay flat and maintain shape and then fully absorbs over time
.jpg)
Materials and Clinical Data
Clinically supported technique since 2002 with peer-reviewed published clinical studies.*
The Ventralex™ Hernia Patch is also available with an absorbable barrier featuring Sepra® Technology – Ventralex™ ST.
*D.F. Martin, R.F. Williams, T. Mulrooney, and G.R. Voeller. “Ventralex™ Mesh in Umbilical/Epigastric Hernia Repairs: Clinical Outcomes and Complications.” Hernia. 2008 Aug 12(4) 379-83.
INDICATIONS
The Bard™ Ventralex™ Hernia Patch is intended for use in all forms of hernia repair requiring reinforcement with a non absorbable support material. The small Bard® Ventralex™ Hernia Patch (4.3 cm/1.7 in) is also intended to repair soft tissue deficiencies, including deficiencies caused by trocars.
CONTRAINDICATIONS
Do not use the Bard™ Ventralex™ Hernia Patch in infants or children, whereby future growth will be compromised by use of such mesh material. Do not use the Bard™ Ventralex™ Hernia Patch for the reconstruction of cardiovascular defects. Literature reports that there is a possibility for adhesion formation when the polypropylene is placed in contact with the bowel or viscera.
WARNINGS
Do not cut or reshape any portion of the Bard® Ventralex™ Hernia Patch (as this could affect its effectiveness), except for the monofilament polypropylene positioning strap. Care should be taken not to cut or nick the SorbaFlex™ PDO Monofilament. If the recoil ring is cut or damaged during insertion or fixation, additional complications may include bowel or skin perforation and infection. Follow proper rolling techniques for all patches as described in these instructions for use as other rolling techniques may potentially compromise the SorbaFlex™ PDO Monofilament. Ensure proper orientation; the solid white surface (ePTFE) must be oriented against the bowel or sensitive organs. Do not place the mesh surface against the bowel. There is a possibility for adhesion formation when mesh (including strap) is placed in direct contact with the bowel or viscera.
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematoma, inflammation, extrusion, fistula formation, infection, allergic reaction, and recurrence of the hernia or soft tissue defect. If the SorbaFlex™ PDO monofilament is cut or damaged during insertion or fixation, additional complications may include bowel or skin perforation and infection.
Please consult package insert for more detailed safety information and instructions for use.
*D.F. Martin, R.F. Williams, T. Mulrooney, and G.R. Voeller. “Ventralex™ Mesh in Umbilical/Epigastric Hernia Repairs: Clinical Outcomes and Complications.” Hernia. 2008 Aug 12(4) 379-83.
INDICATIONS
The Bard™ Ventralex™ Hernia Patch is intended for use in all forms of hernia repair requiring reinforcement with a non absorbable support material. The small Bard® Ventralex™ Hernia Patch (4.3 cm/1.7 in) is also intended to repair soft tissue deficiencies, including deficiencies caused by trocars.
CONTRAINDICATIONS
Do not use the Bard™ Ventralex™ Hernia Patch in infants or children, whereby future growth will be compromised by use of such mesh material. Do not use the Bard™ Ventralex™ Hernia Patch for the reconstruction of cardiovascular defects. Literature reports that there is a possibility for adhesion formation when the polypropylene is placed in contact with the bowel or viscera.
WARNINGS
Do not cut or reshape any portion of the Bard® Ventralex™ Hernia Patch (as this could affect its effectiveness), except for the monofilament polypropylene positioning strap. Care should be taken not to cut or nick the SorbaFlex™ PDO Monofilament. If the recoil ring is cut or damaged during insertion or fixation, additional complications may include bowel or skin perforation and infection. Follow proper rolling techniques for all patches as described in these instructions for use as other rolling techniques may potentially compromise the SorbaFlex™ PDO Monofilament. Ensure proper orientation; the solid white surface (ePTFE) must be oriented against the bowel or sensitive organs. Do not place the mesh surface against the bowel. There is a possibility for adhesion formation when mesh (including strap) is placed in direct contact with the bowel or viscera.
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematoma, inflammation, extrusion, fistula formation, infection, allergic reaction, and recurrence of the hernia or soft tissue defect. If the SorbaFlex™ PDO monofilament is cut or damaged during insertion or fixation, additional complications may include bowel or skin perforation and infection.
Please consult package insert for more detailed safety information and instructions for use.
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
*D.F. Martin, R.F. Williams, T. Mulrooney, and G.R. Voeller. “Ventralex™ Mesh in Umbilical/Epigastric Hernia Repairs: Clinical Outcomes and Complications.” Hernia. 2008 Aug 12(4) 379-83.
INDICATIONS
The Bard™ Ventralex™ Hernia Patch is intended for use in all forms of hernia repair requiring reinforcement with a non absorbable support material. The small Bard® Ventralex™ Hernia Patch (4.3 cm/1.7 in) is also intended to repair soft tissue deficiencies, including deficiencies caused by trocars.
CONTRAINDICATIONS
Do not use the Bard™ Ventralex™ Hernia Patch in infants or children, whereby future growth will be compromised by use of such mesh material. Do not use the Bard™ Ventralex™ Hernia Patch for the reconstruction of cardiovascular defects. Literature reports that there is a possibility for adhesion formation when the polypropylene is placed in contact with the bowel or viscera.
WARNINGS
Do not cut or reshape any portion of the Bard® Ventralex™ Hernia Patch (as this could affect its effectiveness), except for the monofilament polypropylene positioning strap. Care should be taken not to cut or nick the SorbaFlex™ PDO Monofilament. If the recoil ring is cut or damaged during insertion or fixation, additional complications may include bowel or skin perforation and infection. Follow proper rolling techniques for all patches as described in these instructions for use as other rolling techniques may potentially compromise the SorbaFlex™ PDO Monofilament. Ensure proper orientation; the solid white surface (ePTFE) must be oriented against the bowel or sensitive organs. Do not place the mesh surface against the bowel. There is a possibility for adhesion formation when mesh (including strap) is placed in direct contact with the bowel or viscera.
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematoma, inflammation, extrusion, fistula formation, infection, allergic reaction, and recurrence of the hernia or soft tissue defect. If the SorbaFlex™ PDO monofilament is cut or damaged during insertion or fixation, additional complications may include bowel or skin perforation and infection.
Please consult package insert for more detailed safety information and instructions for use.