RELATED PRODUCTS NOT AVAILABLE
true
Support
Sales
1.844.8.BD.LIFE (1.844.823.5433)
Thank you for contacting our sales team!
A sales representive will get in touch with you shortly.
Ordering
1.800.556.6756
BDISurgery@bd.com
Onsite Visiting
100 Crossings BoulevardWarwick, Rhode Island 02886United States
Customer Service
1.844.8.BD.LIFE (1.844.823.5433)
Monday to Friday 8:15 AM to 6:00 PM EST
1.800.531.4124
Custserv.davol@bd.com
Shipping a Product Back
100 Crossings Boulevard, Warwick, Rhode Island 02886, United States
Phasix™ Mesh
Fully Absorbable Mesh for Ventral Hernia Repair and Soft Tissue Reconstruction

- Overview
- Products & Accessories
- EIFU & Resources
- Reimbursement
- FAQs
THE NEXT PHASE IN HERNIA REPAIR
High repair strength through the critical healing phase
Composed of biologically-derived material, Poly-4-hydroxybutyrate (P4HB), Phasix™ Mesh provides critical strength during the initial healing phase with rapid tissue ingrowth and vascularization through its open-pore monofilament structure. The open monofilament mesh scaffold also provides early integration and repair strength*
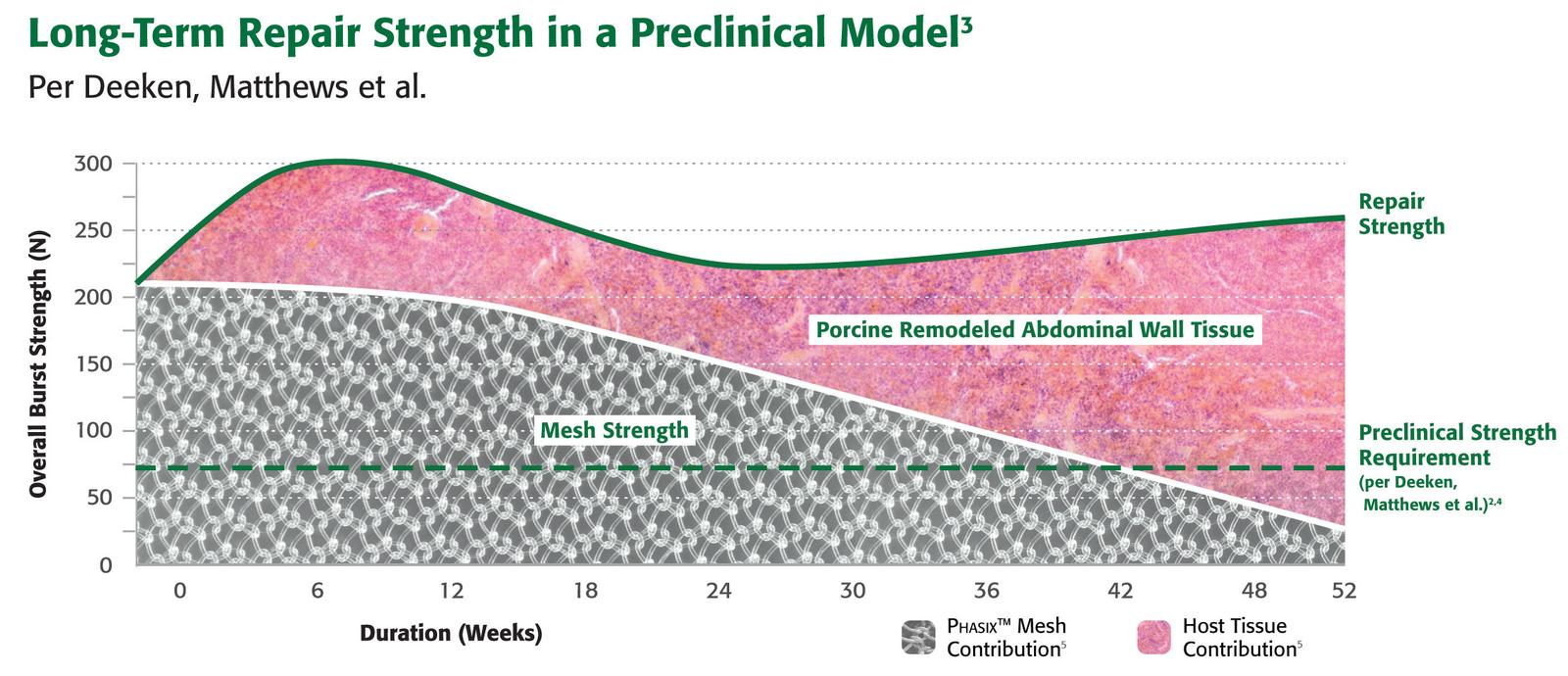
ORGANIZED AND FUNCTIONAL COLLAGEN AT HERNIA REPAIR SITE
Preclinical testing confirms vascular integration and incorporation, with abundant mature collagen at 52 weeks. Gradually transfers load to native tissue over time*
Rapid tissue incorporation
As Phasix™ Mesh is remodeled, it is replaced with functional tissue, ultimately resulting in a strong repair at one year*
Predictable strength for the long run
Phasix™ Mesh gradually and predictably degrades within 12 to 18 months via hydrolysis leaving behind a durable, functional repair with over 3x the strength of the native abdominal wall.
WHAT IS PHASIX™ MESH?
- Phasix™ Mesh is a knitted monofilament mesh scaffold using Poly-4-hydroxybutyrate (P4HB), a biologically derived, fully resorbable material.
- Monomer form (4HB) is a naturally occurring human metabolite found in the brain, heart, liver, kidney, and muscle
- Predictably resorbs through hydrolysis, as P4HB metabolizes into biocompatible byproducts (CO2 and H2O)4
- Phasix™ Mesh has not been shown to break down in the presence of bacteria—maintaining 100% of its strength at 56 days —unlike biologic grafts which demonstrate accelerated degradation in the presence of bacteria.
- As shown in the graphic below, there was no presence of bacterial colonization observed in Phasix Mesh™ or Phasix™ ST Mesh 7 days post-inoculation in preclinical testing. The other side of this graph shows the Presence of abscess (white material) observed SurgiMend®, Strattice™, Bio - A ®, and OviTex™. Other observed indications of bacterial colonization included swelling, the presence of fluids, and thickened capsule tissue.
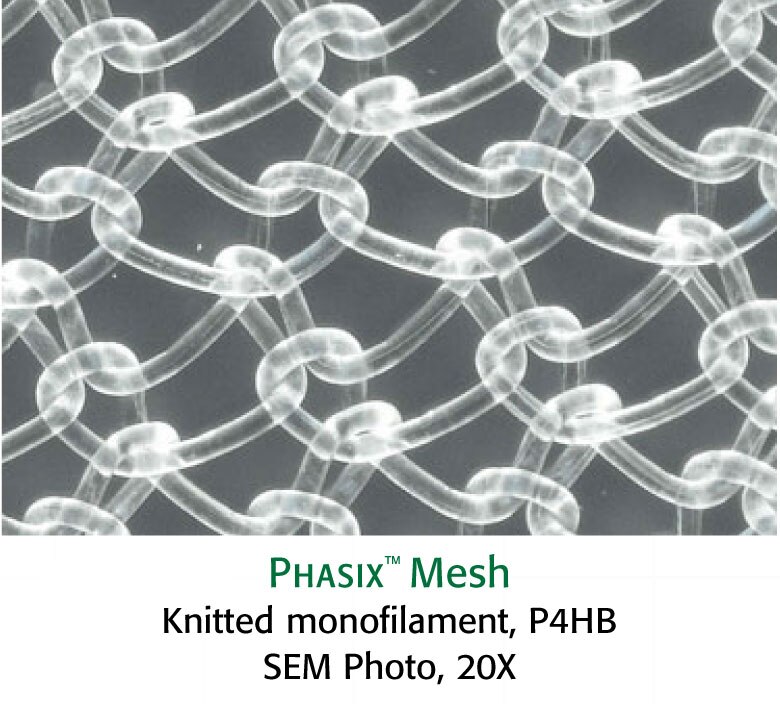
MATERIAL STRUCTURE1
- Material structure can impact host response.5 Consider these features of monofilament versus multifilament structures:
- Monofilament mesh design allows for a prompt fibroblastic response through the open interstices of the mesh1
- Material designs with complex architecture can have greater surface area and niches that bacteria can use as a haven from tissue ingrowth, neovascularization, antibiotic treatment, and host inflammatory response6
- It has been reported that the surface area of multifilament material is 157% higher than monofilament materials6
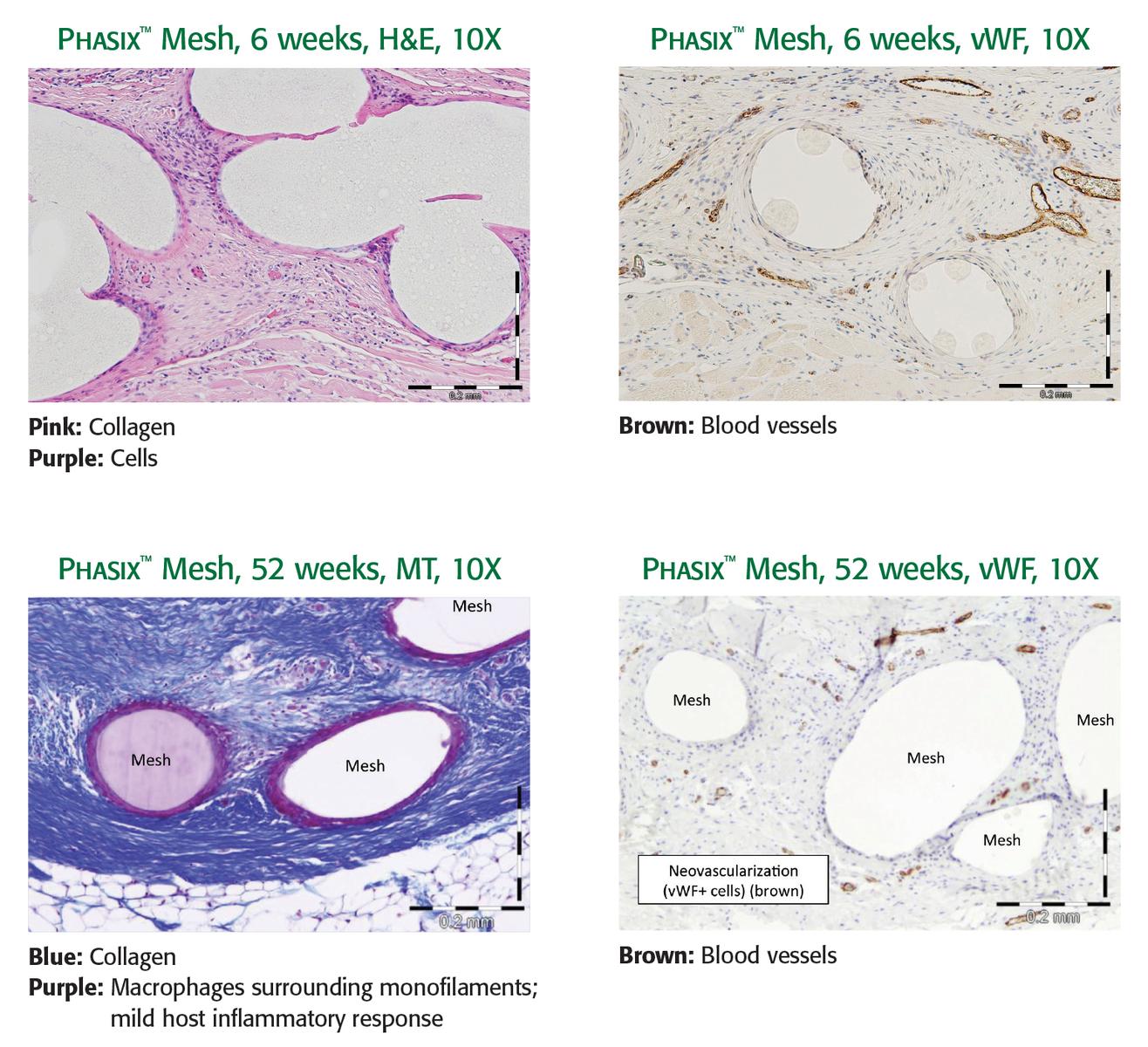
TISSUE INCORPORATION1
- Study objective: Evaluate material strength and histopathology of Phasix™ Mesh.
- Study design: A 3-centimeter round defect was created in the ventral abdominal wall of 25 Yucatan mini-pigs (average weight 38 kg). Phasix™ Mesh was fixated directly over the defect with SorbaFix™ resorbable tacks. Ball burst testing and histopathology were conducted at 6, 12, 26, and 52 weeks.
RESULTS
- Early tissue ingrowth, vascular integration, and incorporation of Phasix™ Mesh into the ventral abdominal wall, plus abundant mature collagen formed around the remaining fibers at 52 weeks.

CLINICAL
Prospective Multi-institutional Evaluation of Phasix™ Mesh in High Risk Ventral and Incisional Hernia Repairs: 18 month follow up. (Click on image to expand).
PROVEN PERFORMANCE FROM A RELIABLE PARTNER
With over 143,000 implants across the Phasix™ Mesh family,11 BD is proud to be the category leader for hernia repair and bioresorbable mesh. BD is committed to providing an innovative hernia portfolio that focuses on improving clinical outcomes for better patient care.
The Phasix™ Mesh family is reliable and used around the globe:
- Over 143,000 implants
- More than 10 clinical studies
- More than 950 patients studied
- Proven clinical outcomes at 3 years
The Phasix™ Mesh family also offers the following benefits
- Low recurrence
- Lower post operative infections
- Low seroma rates
- Associated with improved quality of life
1. Preclinical data on file. Results may not correlate to clinical performance in humans.
2. Deeken CR, Abdo MS, Frisella MM, Matthews BD. “Physicomechanical evaluation of absorbable and nonabsorbable barrier composite meshes for laparoscopic ventral hernia repair.” Surgical Endoscopy 25.5 (2010): 1541-552.
3. Estimated from Standard Curve in (Martin DP, et al. “Characterizationof poly-4-hydroxybutyrate mesh for hernia repair applications.” Journal of Surgical Research. 2013; (184): 766-773
4. Martin, DP. et al. “ Medical applications of poly-4-hydroxybutyrate:a strong flexible absorbable biomaterial. Biochemical Engineering Journal. 2002; December 9
5. Amid PK, Shulman AG, Lichtenstein IL, Hakaha M. Biomaterials for Abdominal Wall Hernia Surgery and Principles of their Applications. Langenbecks Archive Chir. 1994; 379(3): 168-71.
6. Halaweish I, Harth K, Broome AM, Voskerician G, Jacobs MR, Rosen M. Novel In Vitro Model for Assessing Susceptibility of Synthetic Hernia Repair Meshes to Staphylococcus aureus Infection Using Green Fluorescent Protein-Labeled Bacteria and Modern Imaging Techniques. J Surg Infect (Larchmt). 2010; Oct1(5): 449-54.
Indications
Phasix™ Mesh is indicated to reinforce soft tissue where weakness exists in patients undergoing plastic and reconstructive surgery, or for use in procedures involving soft tissue repair, such as the repair of hernia or other fascial defects that require the addition of a reinforcing or bridging material to obtain the desired surgical result.
Contraindications
Because Phasix™ Mesh is fully resorbable, it should not be used in repairs where permanent wound or organ support from the mesh is required.
Warnings
Phasix™ Mesh must not be put in direct contact with bowel or viscera. The safety and product use for patients with hypersensitivities to tetracycline hydrochloride or kanamycin sulfate is unknown. Use of this device in patients with known allergies to these antibiotics should be avoided. The safety and effectiveness of Phasix™ Mesh in pediatric use has not been evaluated or established. If an infection develops, treat the infection aggressively. An unresolved infection may require removal of the device.
Adverse Reactions
Possible complications include infection, seroma, pain, mesh migration, wound dehiscence, hemorrhage, adhesions, hematoma, inflammation, allergic reaction, extrusion, erosion, fistula formation and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.
true
BD-76012
1. Preclinical data on file. Results may not correlate to clinical performance in humans.
2. Deeken CR, Abdo MS, Frisella MM, Matthews BD. “Physicomechanical evaluation of absorbable and nonabsorbable barrier composite meshes for laparoscopic ventral hernia repair.” Surgical Endoscopy 25.5 (2010): 1541-552.
3. Estimated from Standard Curve in (Martin DP, et al. “Characterizationof poly-4-hydroxybutyrate mesh for hernia repair applications.” Journal of Surgical Research. 2013; (184): 766-773
4. Martin, DP. et al. “ Medical applications of poly-4-hydroxybutyrate:a strong flexible absorbable biomaterial. Biochemical Engineering Journal. 2002; December 9
5. Amid PK, Shulman AG, Lichtenstein IL, Hakaha M. Biomaterials for Abdominal Wall Hernia Surgery and Principles of their Applications. Langenbecks Archive Chir. 1994; 379(3): 168-71.
6. Halaweish I, Harth K, Broome AM, Voskerician G, Jacobs MR, Rosen M. Novel In Vitro Model for Assessing Susceptibility of Synthetic Hernia Repair Meshes to Staphylococcus aureus Infection Using Green Fluorescent Protein-Labeled Bacteria and Modern Imaging Techniques. J Surg Infect (Larchmt). 2010; Oct1(5): 449-54.
Indications
Phasix™ Mesh is indicated to reinforce soft tissue where weakness exists in patients undergoing plastic and reconstructive surgery, or for use in procedures involving soft tissue repair, such as the repair of hernia or other fascial defects that require the addition of a reinforcing or bridging material to obtain the desired surgical result.
Contraindications
Because Phasix™ Mesh is fully resorbable, it should not be used in repairs where permanent wound or organ support from the mesh is required.
Warnings
Phasix™ Mesh must not be put in direct contact with bowel or viscera. The safety and product use for patients with hypersensitivities to tetracycline hydrochloride or kanamycin sulfate is unknown. Use of this device in patients with known allergies to these antibiotics should be avoided. The safety and effectiveness of Phasix™ Mesh in pediatric use has not been evaluated or established. If an infection develops, treat the infection aggressively. An unresolved infection may require removal of the device.
Adverse Reactions
Possible complications include infection, seroma, pain, mesh migration, wound dehiscence, hemorrhage, adhesions, hematoma, inflammation, allergic reaction, extrusion, erosion, fistula formation and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.
true
BD-16443
Literature
BD's collection of literature on industry and on our offerings gives you information you can use to continue striving for excellence.
Learn more
Training
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
Learn more
Events
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
Learn more
Case Studies
BD promotes clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.
Learn more
1. Preclinical data on file. Results may not correlate to clinical performance in humans.
2. Deeken CR, Abdo MS, Frisella MM, Matthews BD. “Physicomechanical evaluation of absorbable and nonabsorbable barrier composite meshes for laparoscopic ventral hernia repair.” Surgical Endoscopy 25.5 (2010): 1541-552.
3. Estimated from Standard Curve in (Martin DP, et al. “Characterizationof poly-4-hydroxybutyrate mesh for hernia repair applications.” Journal of Surgical Research. 2013; (184): 766-773
4. Martin, DP. et al. “ Medical applications of poly-4-hydroxybutyrate:a strong flexible absorbable biomaterial. Biochemical Engineering Journal. 2002; December 9
5. Amid PK, Shulman AG, Lichtenstein IL, Hakaha M. Biomaterials for Abdominal Wall Hernia Surgery and Principles of their Applications. Langenbecks Archive Chir. 1994; 379(3): 168-71.
6. Halaweish I, Harth K, Broome AM, Voskerician G, Jacobs MR, Rosen M. Novel In Vitro Model for Assessing Susceptibility of Synthetic Hernia Repair Meshes to Staphylococcus aureus Infection Using Green Fluorescent Protein-Labeled Bacteria and Modern Imaging Techniques. J Surg Infect (Larchmt). 2010; Oct1(5): 449-54.
Indications
Phasix™ Mesh is indicated to reinforce soft tissue where weakness exists in patients undergoing plastic and reconstructive surgery, or for use in procedures involving soft tissue repair, such as the repair of hernia or other fascial defects that require the addition of a reinforcing or bridging material to obtain the desired surgical result.
Contraindications
Because Phasix™ Mesh is fully resorbable, it should not be used in repairs where permanent wound or organ support from the mesh is required.
Warnings
Phasix™ Mesh must not be put in direct contact with bowel or viscera. The safety and product use for patients with hypersensitivities to tetracycline hydrochloride or kanamycin sulfate is unknown. Use of this device in patients with known allergies to these antibiotics should be avoided. The safety and effectiveness of Phasix™ Mesh in pediatric use has not been evaluated or established. If an infection develops, treat the infection aggressively. An unresolved infection may require removal of the device.
Adverse Reactions
Possible complications include infection, seroma, pain, mesh migration, wound dehiscence, hemorrhage, adhesions, hematoma, inflammation, allergic reaction, extrusion, erosion, fistula formation and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.
true
BD-16443
The BD Phasix™ Reimbursement Hotline is available to answer your questions.
The hotline can be reached via phone or email.
(800)614-7965 | reimbursementsupport@bd.com
Any email or voice mail will be returned within 48 business hours. For information on physician billing, please contact your respective professional society.
Phasix™ Mesh provides a fully resorbable monofilament scaffold for rapid tissue incorporation that has been designed to allow for the repair strength of a synthetic mesh along with the remodeling characteristics of a biologic graft.
Composed of biologically-derived material, Poly-4-hydroxybutyrate (P4HB), Phasix™ Mesh provides critical strength during the initial healing phase with rapid tissue ingrowth and vascularization through its open-pore monofilament structure. Monomer form (4HB) is a naturally occurring human metabolite found in the brain, heart, liver, kidney, and muscle and P4HB scaffold has been used clinically for hernia repair for more than 5 years.
Phasix™ Mesh gradually and predictably degrades within 12 to 18 months via hydrolysis leaving behind a durable, functional repair with over 3x the strength of the native abdominal wall.
Phasix™ Mesh has not been shown to break down in the presence of bacteria - maintaining 100% of its strength at 56 days - unlike biologic grafts which demonstrate accelerated degradation in the presence of bacteria. Additionally, Phasix™ Mesh offers the benefits of rapid tissue incorporation with organized and functional collagen at the repair site, while offering high repair strength through the critical healing phase.
Phasix™ ST Mesh combines two market-leading technologies into one product: monofilament resorbable Phasix™ Mesh and a proven hydrogel barrier based on Sepra® technology. It handles, sutures and fixates like a synthetic mesh, while facilitating trocar deployment during laparoscopic placement.
Phasix™ ST Mesh combines Phasix™ Mesh and a proven hydrogel barrier based on Sepra® technology into one product. While the monofilament mesh supports functional healing and a strong repair, the hydrogel barrier is designed to minimize tissue attachment to the visceral side of the mesh for intraabdominal placement.
Phasix™ ST Mesh is a biologically derived scaffold with a hydrogel barrier for intraabdominal placement. It has been designed to provide the repair strength of a synthetic mesh and the remodeling characteristics of a biologic. Additionally, its longitudinal stripes aid with orientation and visibility during placement.
The ST hydrogel barrier is designed to minimize the risk of tissue attachment, resorbs within 30 days, and has over 10 years of clinical application.
Phasix™ Mesh comes in the following sizes:
- 6" x 12" Rectangle (15 x 30 cm)
- 8" x 10" Rectangle (20.3 x 25.4 cm)
- 8" x 12" Rectangle (20 x 30 cm)
- 8" x 16" Rectangle (20 x 40 cm)
- 10" x 12" Rectangle (25.4 x 30.5 cm)
- 12" x 12" Square (30 x 30 cm)
- 10" x 16" Rectangle (25 x 40 cm)
- 12" x 18" Rectangle (30 x 45 cm)
- 14" x 14" Square (35 x 35 cm)
- 16" x 16" Square (40 x 40 cm)
- 18" x 18" Square (45 x 45 cm)
- 19.5" x 19.5" Square (50 x 50 cm)
- 3”x 3" Round (7.6 cm Round)
- 4”x 6” Rectangle (10.2 cm x 15.2 cm)
- 4.5" Round (11 cm)
- 3" x 3" Square (8 x 8 cm)
- 2.4" x 6.3" Rectangle (6 x 16 cm)
- 3" x 6.3" Rectangle (8 x 16 cm)
- 3" x 8" Rectangle (8 x 20 cm)
- 4" x 4" Square (10 x 10 cm)
- 4" x 8" Rectangle (10 x 20 cm)
- 4" x 10" Rectangle (10 x 25 cm)
- 6" x 8" Rectangle (15.2 x 20.3 cm)
- 6" x 10" Rectangle (15 x 25 cm)
- 8" x 8" Square (20 x 20 cm)
Phasix™ Mesh comes in the following sizes:
- Round 3" (8 cm)
- Round 4.5" (11 cm)
- Round 6" (15 cm)
- Rectangle 3" x 4" (7 cm x 10 cm)
- Square 4" x 4" (10 cm x 10 cm)
- Rectangle 4" x 6" (10 cm x 15 cm)
- Rectangle 4" x 8" (10 cm x 20 cm)
- Rectangle 5" x 10" (13 cm x 25 cm)
- Rectangle 6" x 8" (15 cm x 20 cm)
- Rectangle 8" x 10" (20 cm x 25 cm)
- Rectangle 10" x 12" (25 cm x 30 cm)
- Rectangle 12" x 14" (30 cm x 35 cm)
More than 10 clinical studies with more than 950 patients studied
Phasix Clinical Compendium
For more information, please contact your local sales rep to learn more
2. Internal market research, data on file, 2015.
3. Liang MK, Li LT, Nguyen MT, Berger RL, Hicks SC, Kao LS. Abdominal reoperation and mesh explantation following open ventral hernia repair with mesh. The American Journal of Surgery. 208.4(2014):670–76.
4. Itani KM, et al. Prospective study of single-stage repair of contaminated hernias using a biologic porcine tissue matrix: the RICH Study. Surgery. 2012;152(3):498–505.
5. Annor AH, Tang ME, Pui CL, Ebersole GC, Frisella MM, Matthews BD, Deeken CR. Effect of enzymatic degradation on the mechanical properties of biological scaffold materials. Surgical Endoscopy. 26.10(2012):2767–778. 6. Harth KC, et al. Effect of surgical wound classification on biologic graft performance in complex hernia repair: an experimental study. Surgery. 2013;153(4):481–492
BD-46830
true
true