true
BD PhaSeal™ System
Protect healthcare workers from exposure to hazardous drugs with a closed system drug transfer device
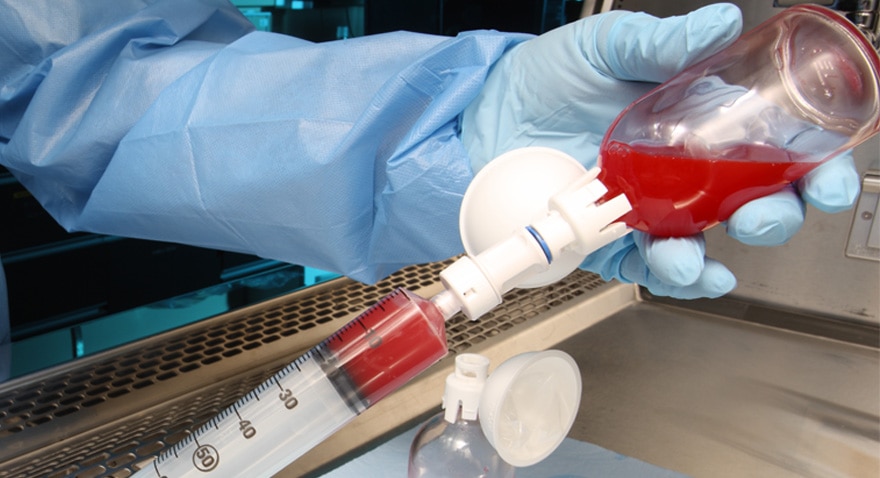
BD PhaSeal™ System
Ready to learn more? Let’s have a conversation.
- Overview
- Products & Accessories
- EIFU & Resources
Reference
*Within an ISO Class V environment following aseptic technique
†The ability to prevent microbial ingress for up to 168 hours should not be interpreted as modifying, extending, or superseding a manufacturer’s labeling recommendations for the storage and expiration dating of the drug vial. Refer to the drug manufacturer’s recommendations and USP compounding guidelines for shelf life and sterility information.
BD-113232 (1/24)
Resources
Literature
BD's collection of literature on industry and on our offerings gives you information you can use to continue striving for excellence.
Learn more
true
true