IV Care and Maintenance
BD offers a full portfolio of IV care and maintenance products that provide clinicians with the tools they need to help prevent catheter dislodgement and site contamination.
-

BD ChloraPrep™ is a broad-spectrum, rapid-acting patient preoperative skin preparation with persistent antimicrobial activity for at least 7 days.
-

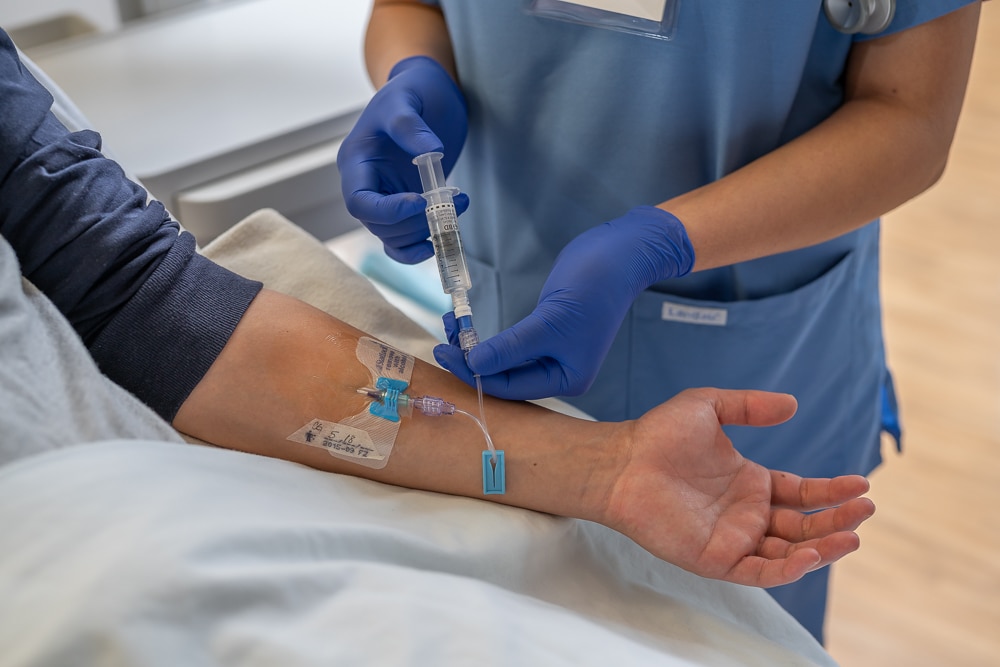
The BD StatLock™ Stabilization Device portfolio ensures that healthcare professionals have the correct catheter stabilization solution for virtually any vascular access device used by their facility.
-

The Securis™ Stabilization Device was designed to secure non-winged PIVC catheters and minimize skin irritation.
-

GuardIVa™ Dressing is an antimicrobial dressing that is also indicated to control surface bleeding at insertion sites.
-

The MaxPlus™ and MaxZero™ Needle-free Connector technology is designed to help reduce the risk of infections and occlusions.
-

The Q-Syte™ Needle-free Connector has a split septum design that adheres to CDC and INICC guidelines for reducing CRBSIs.
-

The SmartSite™ needle-free valve provides a wide variety of benefits in IV therapy, including helping prevent infection.
-

The PureHub™ Disinfecting Cap acts as a physical barrier between line accesses and is indicated as a disinfecting device for swabbable needle-free luer connectors prior to IV access.
-

Site-Scrub™ IPA Device disinfects injection ports and female luer hubs through a friction scrub device with 70% IPA designed to disinfect catheter hubs with a 10-second scrub
-

PosiFlush™ Pre-filled Flush Syringes are designed to help reduce the risk of medication errors and may help lower the risk of catheter damage and reduce disposal waste.
-
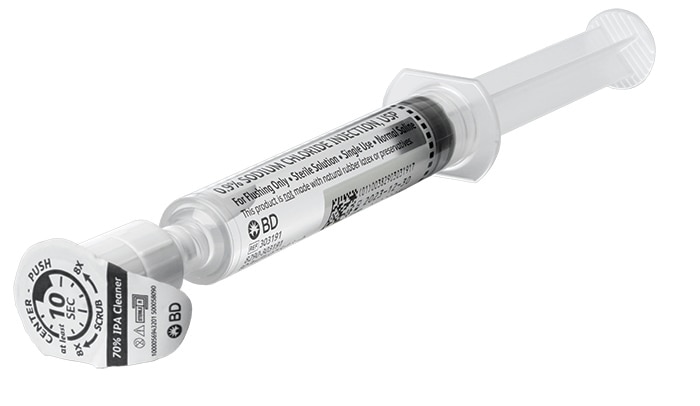
With an active disinfection unit built into the tip of the syringe, the all-in-one PosiFlush™ SafeScrub Prefilled Saline Flush Syringe is demonstrated to significantly reduce microbial growth associated with potentially serious CRBSIs.
BD-117585 (05/24)