1. Millikan KW, Doolas A. A Long-Term Evaluation of the Modified Mesh-Plug Hernioplasty in Over 2,000 Patients. Hernia. 2008 June; 12(3): 257-260.
INDICATIONS
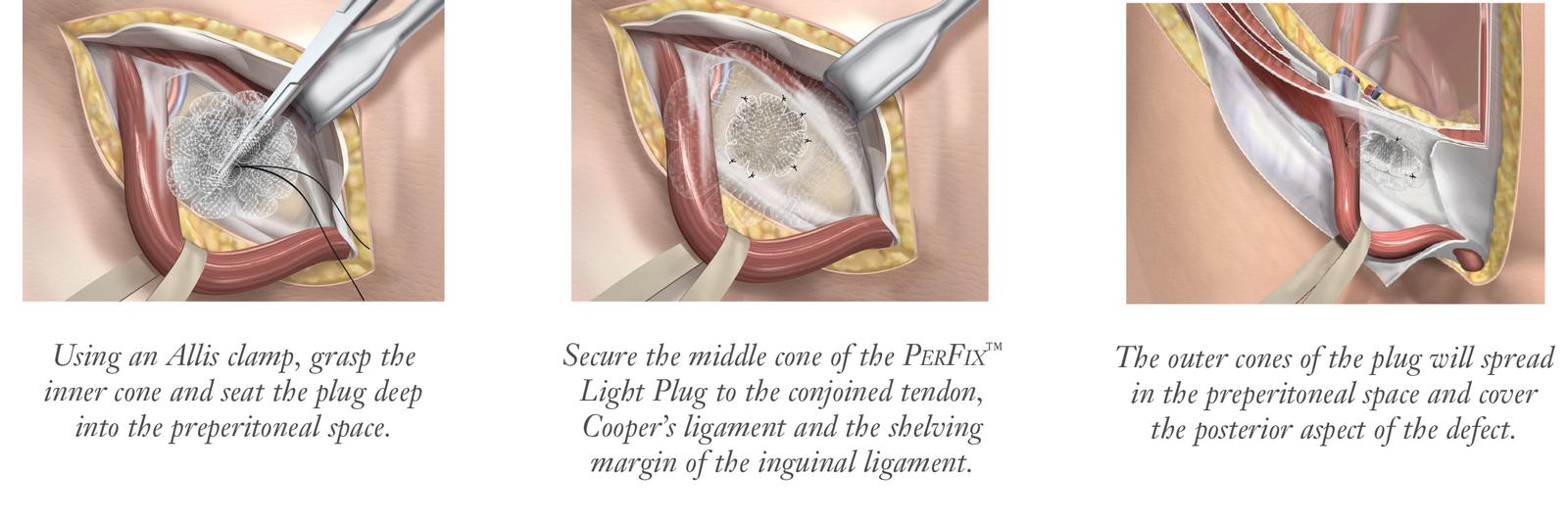
The PerFix™ Light Plug is indicated for reinforcement of soft tissue, where weakness exists, in procedures involving soft tissue repair, such as groin hernia defects.
CONTRAINDICATIONS
Literature reports that there may be a possibility for adhesion formation when polypropylene mesh is placed in direct contact with the bowel or viscera.
Do not use the PerFix™ Light Plug in infants or children, whereby future growth will be compromised by use of such mesh material.
WARNINGS
The use of any permanent mesh or patch in a contaminated or infected wound could lead to fistula formation and/or extrusion of the mesh.
If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require removal of the device.
PRECAUTIONS
Monofilament sutures are recommended to properly secure the PerFix™Light Plug
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematoma, inflammation, extrusion, infection, pain, mesh migration, fistula formation and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use
.