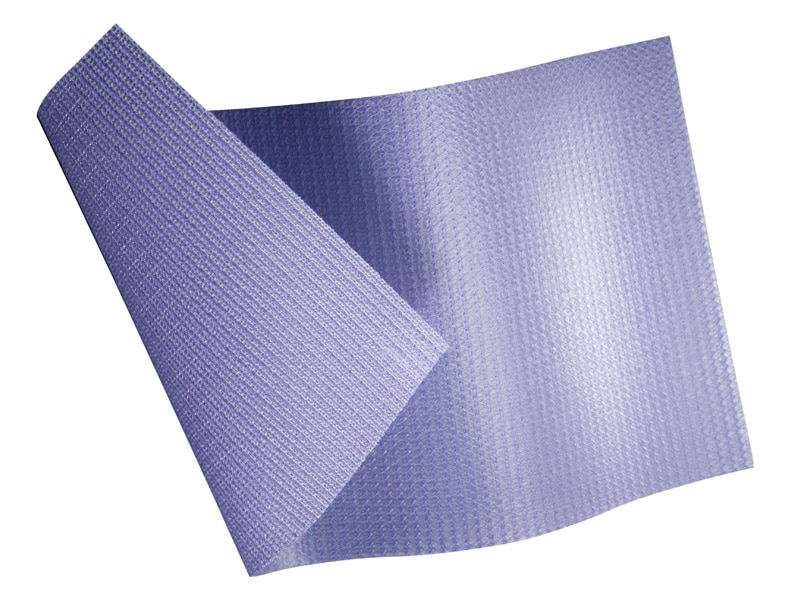
Composix™ E/X Mesh
Polypropylene/ePTFE prosthesis for laparoscopic ventral hernia repair.
- Overview
- EIFU & Resources
Polypropylene and Permanent ePTFE Barrier Designed for Ventral Hernia Repair
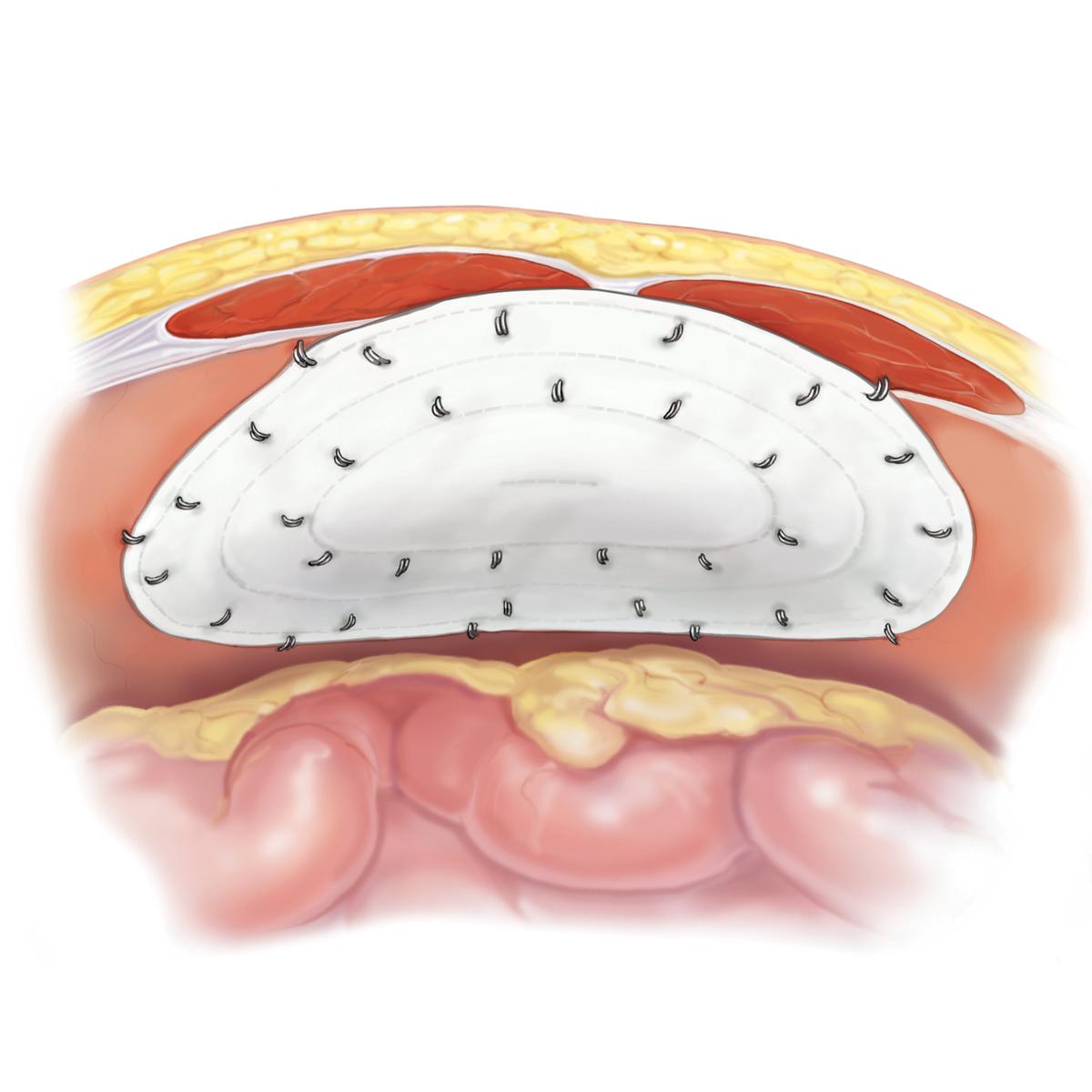
The Bard™ Composix™ E/X Mesh, combines two clinically proven biomaterials, Bard™ polypropylene mesh and ePTFE, into one product. The polypropylene side of the Bard™ Composix™ E/X Mesh promotes tissue ingrowth into the abdominal wall over time and eliminates the need for transfixation sutures. The visceral side of the Bard™ Composix™ E/X Mesh features a permanent submicronic ePTFE barrier, which minimizes attachments to the prosthesis. This ePTFE barrier is permanent, offering long-term protection.
Two distinctly different sides:
- Polypropylene Bard™ mesh provides optimal tissue ingrowth
- Robust sub-micronic ePTFE permanent barrier minimizes attachments to the prosthesis
Elliptically shaped design
Reduces the need to trim the mesh, saving time
Low profile mesh
That is easy to manipulate for open repairs and to deploy for laparoscopic repairs
Monofilament PTFE stitches
Minimize attachments to the prosthesis
Sealed edges
Provide an overhang of ePTFE without compromising the polypropylene Bard® Mesh side
INDICATIONS
Bard™ Composix™ E/X Mesh is indicated for use in the reconstruction of soft tissue deficiencies, such as for the repair of hernias and chest wall defects.
CONTRAINDICATIONS
- Literature reports there may be a possibility for adhesion formation when the polypropylene is placed in direct contact with the bowel or viscera.
- Do not use the Bard™ Composix™ E/X Mesh, in infants or children whereby future growth will be compromised by use of such material.
- Do not use Bard™ Composix™ E/X Mesh, for the reconstruction of cardiovascular defects.
WARNINGS
- Ensure proper orientation; the solid white surface (ePTFE) must be oriented against the bowel or sensitive organs. Do not place the polypropylene mesh surface against the bowel or sensitive organs. There may be a possibility for adhesion formation when polypropylene mesh is placed in direct contact with the bowel or viscera.
- If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require removal of the device.
PRECAUTIONS
DAVOL™ fixation devices or nonabsorbable monofilament sutures are recommended to properly secure the prosthesis. If other fixation devices are used, they must be indicated for use in hernia repair. Care should be taken to ensure that the prosthesis is adequately fixated to the abdominal wall. If necessary, additional fasteners and/or sutures should be used.
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.
BD-14808
Our collection of literature on industries and on our offerings gives you information you can use to continue striving for excellence.
Training resources to help improve your clinical practices as part of our goal of advancing the world of health.
We support the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
We promote clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.
INDICATIONS
Bard™ Composix™ E/X Mesh is indicated for use in the reconstruction of soft tissue deficiencies, such as for the repair of hernias and chest wall defects.
CONTRAINDICATIONS
Literature reports there may be a possibility for adhesion formation when the polypropylene is placed in direct contact with the bowel or viscera.
Do not use the Bard™ Composix™ E/X Mesh, in infants or children whereby future growth will be compromised by use of such material.
Do not use Bard™ Composix™ E/X Mesh, for the reconstruction of cardiovascular defects.
WARNINGS
Ensure proper orientation; the solid white surface (ePTFE) must be oriented against the bowel or sensitive organs. Do not place the polypropylene mesh surface against the bowel or sensitive organs. There may be a possibility for adhesion formation when polypropylene mesh is placed in direct contact with the bowel or viscera.
If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require removal of the device.
PRECAUTIONS
DAVOL™ fixation devices or nonabsorbable monofilament sutures are recommended to properly secure the prosthesis. If other fixation devices are used, they must be indicated for use in hernia repair. Care should be taken to ensure that the prosthesis is adequately fixated to the abdominal wall. If necessary, additional fasteners and/or sutures should be used.
ADVERSE REACTIONS
Possible complications include seroma, adhesions, hematomas, inflammation, extrusion, fistula formation and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.