-
Ventralight™ ST Mesh, Ellipse, 8" x 10" (20.3 cm x 25.4 cm)
SKU/REF 5954810g
-
Ventralight™ ST Mesh, Circle, 8" (20.3 cm)
SKU/REF 5954800g
-
Ventralight™ ST Mesh, Ellipse, 7" x 9" (17.8 cm x 22.9 cm)
SKU/REF 5954790g
-
Ventralight™ ST Mesh, Ellipse, 6" x 8" (15.2 cm x 20.3 cm)
SKU/REF 5954680g
-
Ventralight™ ST Mesh, Oval, 6" x 10" (15.2 cm x 25.4 cm)
SKU/REF 5954610g
-
Ventralight™ ST Mesh, Circle, 6" (15.2 cm)
SKU/REF 5954600g
-
Ventralight™ ST Mesh, Ellipse, 4" x 6" (10.2 cm x 15.2 cm)
SKU/REF 5954460g
-
Ventralight™ ST Mesh, Circle, 4.5" (11.4 cm)
SKU/REF 5954450g
-
Ventralight™ ST Mesh, Rectangle, 12" x 14" (30.5 cm x 35.6 cm)
SKU/REF 5954124g
-
Ventralight™ ST Mesh, Ellipse, 10" x 13" (25.4 cm x 33 cm)
SKU/REF 5954113g
Canada
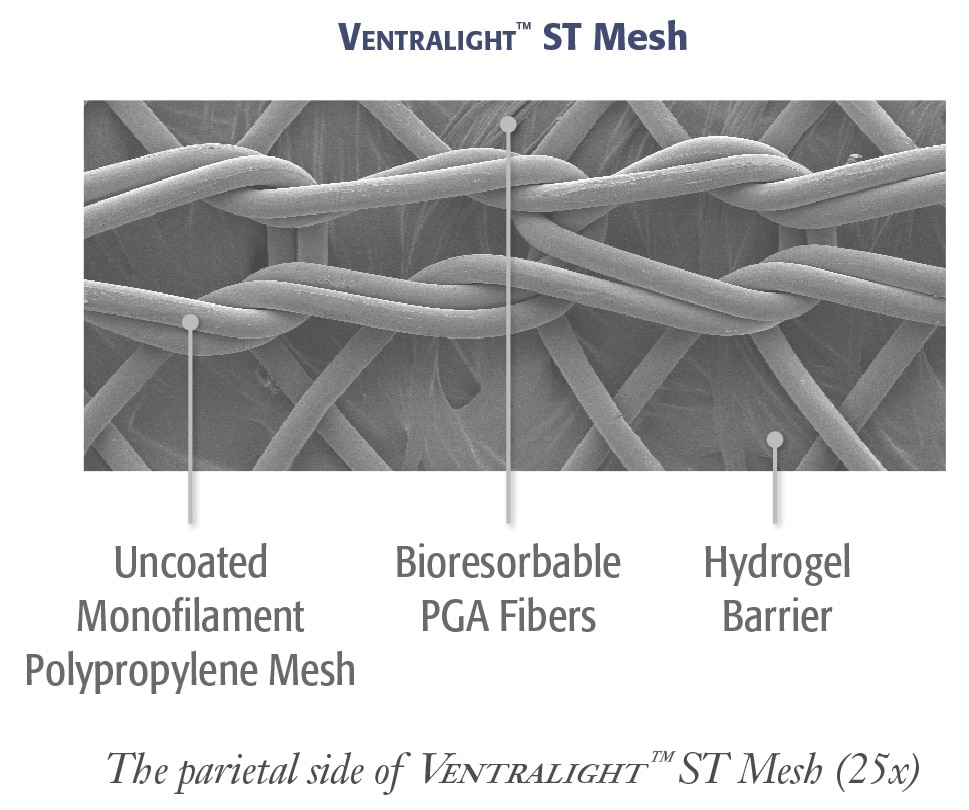
Ventralight™ ST Mesh
Medium weight monofilament polypropylene mesh on the anterior side with an absorbable hydrogel barrier based on Sepra® Technology on the posterior side for laparoscopic ventral hernia repair.


- Overview
- Products & Accessories
- EIFU & Resources
Efficient
- Low profile design facilitates trocar deployment and mechanical fixation.
- Multiple shapes (circle, oval, ellipse, rectangle) and sizes ranging from a 4.5" (11.4 cm) circle to 12" x 14" (30.5 cm x 35.6 cm) rectangle.
- Easily cut to customize shape and size. The unique hydrogel barrier covers the edge of the mesh even after trimming.
Effective
Minimal contraction shown in preclinical testing compared to a leading microporous absorbable barrier mesh
At 4 weeks, Ventralight™ ST Mesh demonstrated 42% less area meshcontracture than a competitive macroporous absorbable barrier mesh.
Results were statistically significant.*
Proven
Ventralight™ Absorbable Barrier based on Sepra® Technology
- Unique hydrogel barrier swells to minimize tissue attachment to the visceral side of the mesh*
- The hydrogel barrier resorbs within 30 days providing visceral protection during the critical healing period*
Strong tissue Proven incorporation
The open-pore design of the uncoated monofilament polypropylene in Ventralight™ ST Mesh allows for:
- Fast tissue ingrowth
- Strong tissue incorporation into the abdominal wall
- A strong, long-term repair uncoated polypropylene allows for the majority of tissue ingrowth and strength to occur in the first two weeks after the placement of a composite hernia prosthesis.**
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.

