Canada
Ventralex™ ST Hernia Patch for Surgery & Repair
A clinically proven umbilical hernia repair solution designed for ventral, incisional, umbilical and epigastric hernia repair as well as trocar site closure, with an absorbable barrier featuring Sepra® technology.

- Overview
- EIFU & Resources

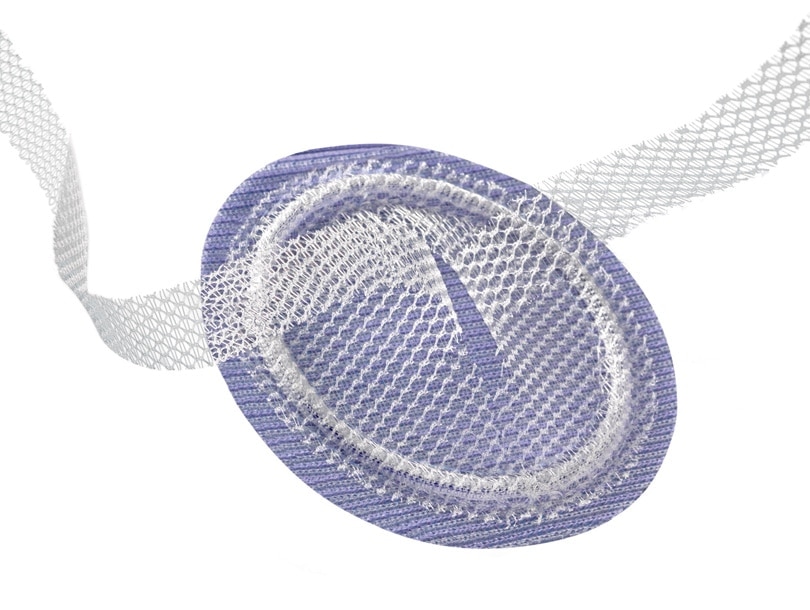
Anterior layer of the Ventralex™ ST Hernia Patch
Uncoated Mesh
Monofilament polypropylene mesh designed to allow a prompt fibroblastic response through the open interstices of the mesh.
- Over 40 years of proven results in hernia repair.
- Allows a fast fibrotic response for a strong repair.
- Provides a long-term repair with minimized recurrence
Designed for a strong hernia repair
SorbaFlex™ Memory Technology
Polydioxanone (PDO) monofilament is unique in its flexibility and tensile strength, facilitating patch insertion and placement. Preclinical testing has demonstrated that absorption via hydrolysis is essentially complete in 6-8 months.4
Unique Pocket and Strap Design
Pocket and strap facilitate placement, positioning and lateral fixation. SorbaFlex™ Memory Technology is contained within a knitted polypropylene mesh tube.
Posterior layer of the hernia patch
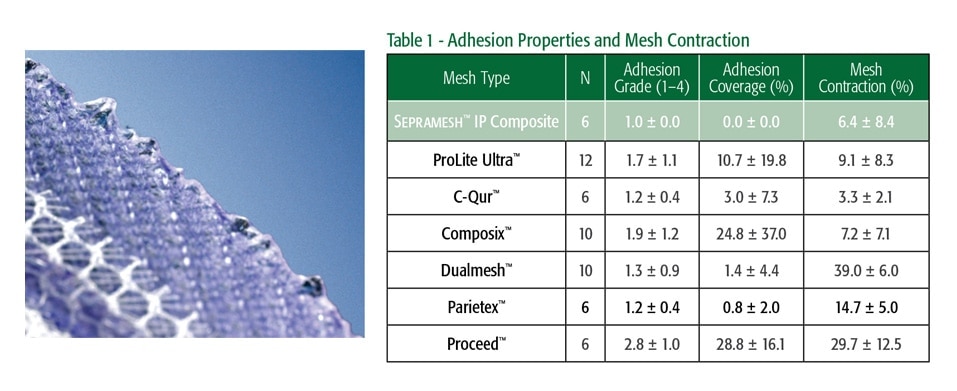
Sepramesh™ IP Composite
The Sepramesh™ IP Composite is a unique hydrogel barrier, based on the Sepra® Technology, swells to minimize tissue attachment to the visceral side of the mesh and resorbs within 30 days providing visceral protection during the critical healing process.3
Bioresorbable PGA fibers reinforce the integrity of the hydrogel barrier by binding it to the polypropylene mesh.
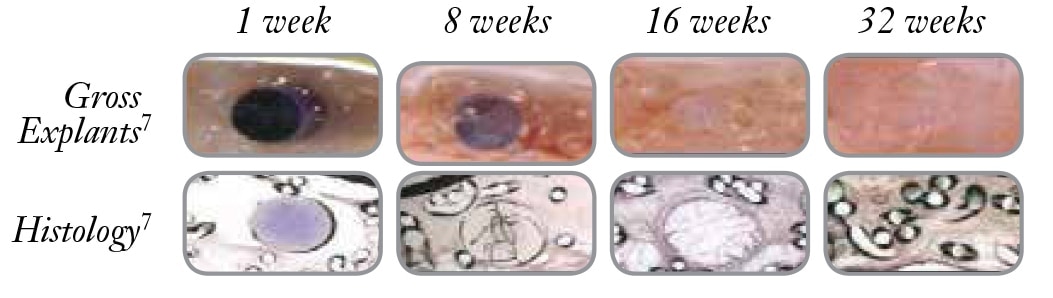
Sepramesh™ IP Composite Preclinical Study2,3
120-Day Comparative Analysis of Adhesion Grade and Quantity, Mesh Contraction, and Tissue Response to a Novel Omega-3 Fatty Acid Bioresorbable Barrier Macroporous Mesh After Intraperitoneal Placement.
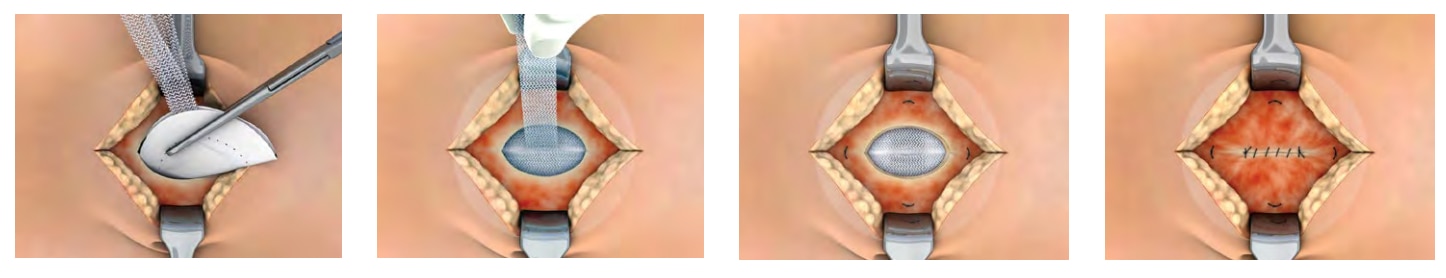
The Ventralex™ ST Hernia Patch Advantage
Efficient Design
- Proprietary pocket and strap design facilitates placement, positioning and lateral fixation.
- SorbaFlex™ Memory Technology allows the patch to “spring open,” lay flat to maintain shape and then fully absorbs over time.1
- Three sizes available for coverage of larger defects to smaller trocar site closures, including the 4.3 cm, the 6.4 cm, and the 8 cm.
- Special positioning strap and memory technology assure that the patch lays flat against the abdominal wall.

Clinical Summary “ Ventralex Mesh in Umbilical/Epigastric Hernia Repairs: Clinical Outcomes and Complications” (Hernia / 2008)
Overview
• 88 patients (69 male, 19 female) were evaluated from 2003-2006 and 89 Ventralex™ Hernia Patches were placed.
• 0 hernia recurrences.
Highlights
“Interrupted nonabsorbable 2-0 Prolene U-stitches are used at the 12 and 6 o’clock position for the 4.3 cm patch and at the 12, 3, 6 and 9 o’clock position for the 6.4 cm patch, attaching only the polypropylene part of the patch to the fascia.”
“We believe our attention to meticulous technique, securing the patch to good healthy fascia at least 2 cm beyond the defect, placement of the patch behind the defect, and re-approximating the fascia over the patch are essential to our low complication and recurrence rate.
Ventralex™ ST Hernia Patch Compared to Parietex™ Ventral Patch
Parietex™ Ventral Patch
Parietex™ Ventral Patch is porcine based and absorbs in 21 days. It has two removal handles composed of colored tubes and yarns and four flaps (one at each corner of the device) made up of bidimensional monofilament polyester. It also has two dyed PGLA poly (glycolide-co-L-lactide) expanders. The PGLA component is completely absorbed prior to one year.
Ventralex™ ST Hernia Patch
Sepramesh™ IP Composite’s unique hydrogel barrier, based on the Sepra® Technology, swells to minimize tissue attachment to the visceral side of the mesh and resorbs within 30 days providing visceral protection during the critical healing process. Sepra® Technology swells over 60% more than Parietex™ Composite barrier. Additionally, monofilament polypropylene mesh allows a prompt fibroblastic response through the open interstices of the mesh.
To read more on the comparison of these two products, check out our in-depth review here.
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.