Canada

- Overview
- EIFU & Resources
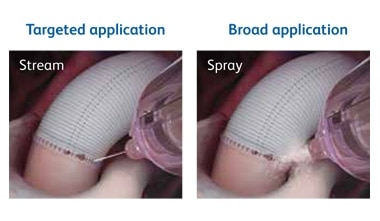
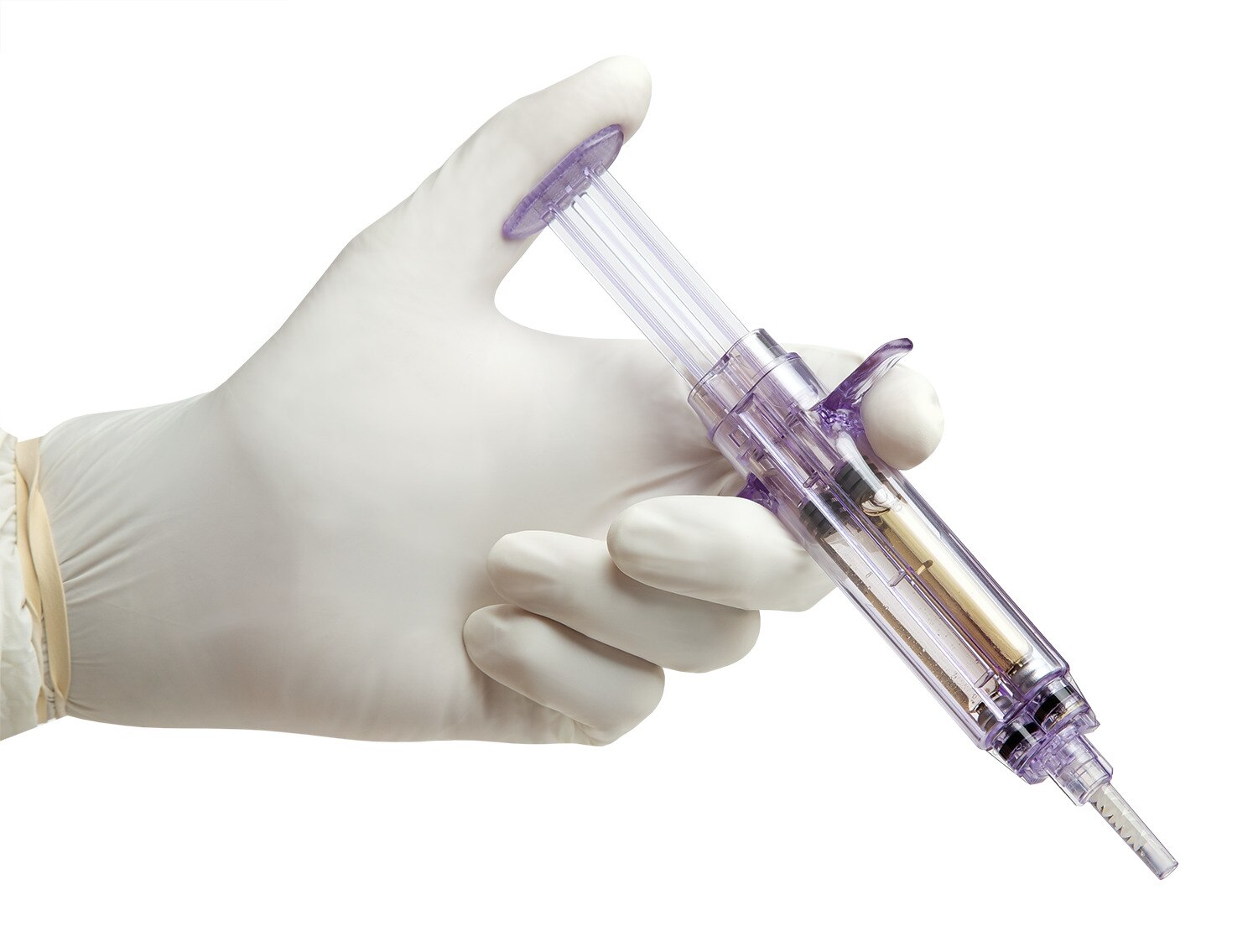
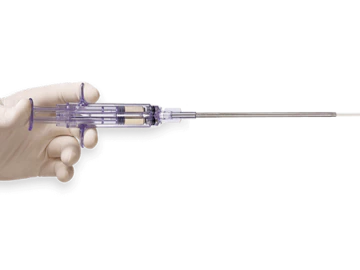
Tridyne™ Vascular Sealant provides application control without the need for pressurized gas.
The Tridyne™ applicator is designed to:
- Provide uniform, continuous coverage without clumping
- Control sealant application by varying hand pressure
- Easily transition from targeted application over the anastomosis to broader coverage to address more diffuse bleeding
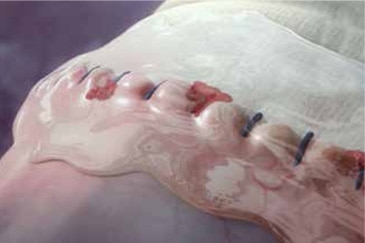
The specialized Tridyne™ formulation is transparent and forms a completely clear hydrogel at the application site. With its unique combination of clarity and coverage, Tridyne™ Vascular Sealant allows for visual confirmation of hemostasis at the anastomotic suture line—making it an ideal choice for aortic surgery.

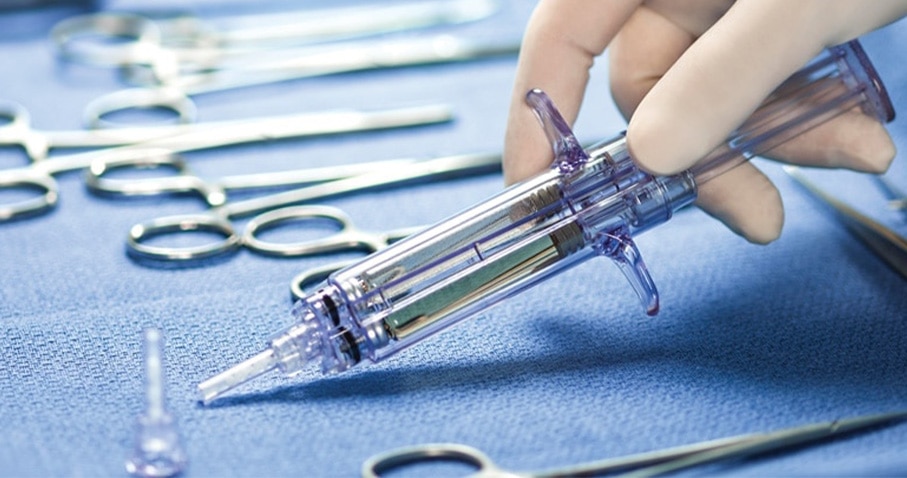
In a prospective, randomized, controlled multicenter study, Tridyne™ Vascular Sealant outperformed Gelfoam® Plus Hemostasis Kit in several critical measures. The study included 156 patients from 18 different centers who underwent thoracic aortic surgery, with hemostasis evaluated at the aortic anastomotic suture line.1
The proven solution during aortic repair1
Tridyne™ Vascular Sealant offers surgeons a specialized, easy-to-use solution during aortic repair, with unique application control and clinically proven results.1 With its demonstrated ability to provide fast, effective control of bleeding, it’s clearly the sealant choice for aortic surgery.
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.