-
PerFix™ Plug, Extra Large, 1.6" x 2.0" (4.1cm x 5.0cm), 2/case
SKU/REF 0112780
-
PerFix™ Plug, Large, 1.6" x 1.90" (4.1cm x 4.8cm), 2/case
SKU/REF 0112770
-
PerFix™ Plug, Medium, 1.3" x 1.55" (3.3cm x 3.9cm), 2/case
SKU/REF 0112760
-
PerFix™ Plug, Small, 1.0" x 1.35" (2.5cm x 3.4cm), 2/case
SKU/REF 0112750
Canada
true
PerFix™ Plug
Plug and patch designed for use in a tension-free open inguinal hernia repair technique.


- Overview
- Products & Accessories
- EIFU & Resources
Efficient
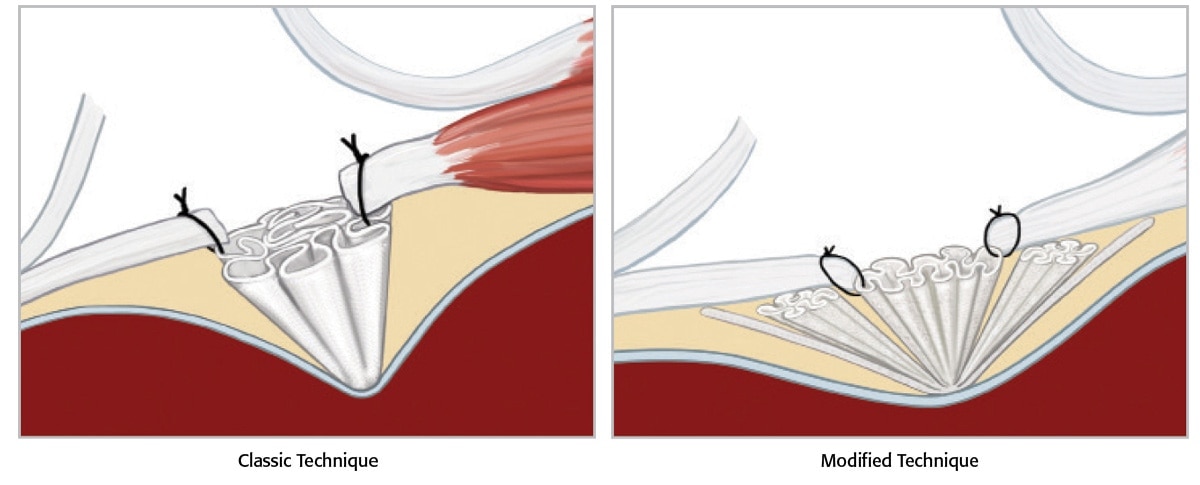
Entire operation can take 25 minutes or less2 Minimal dissection is required 4-5cm incision1 Local or epidural anesthesia can be used Accommodates all types of groin hernias
Effective
Utilized in a tension-free repair technique Recurrence rates reported at 0.15%1 Less than 0.5% chronic pain rate1
Literature
BD's collection of literature on industry and on our offerings gives you information you can use to continue striving for excellence.
Learn more
Training
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
Learn more
Events
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
Learn more
Case Studies
BD promotes clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.
Learn more
true