
Canada
Connect to confidence, care and comfort


BD Cathena™ Safety IV Catheter with BD Multiguard™ Technology elevates the level of safety by combining needlestick safety with blood control technology compared to a non-safety, non-blood control catheter.
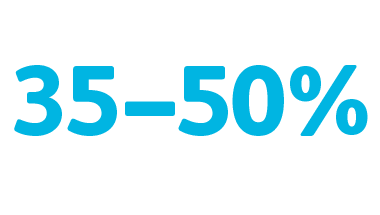
Potentially high frequency of PIV catheter complications
35–50% of hospitalized patients who receive a PIVC experience a catheter-related complication.3
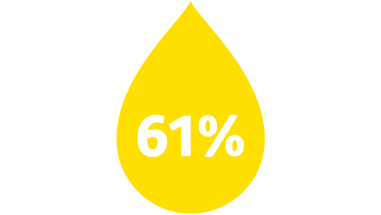
Occurance of blood exposure
In a study, when clinicians used a PIVC without blood control technology, blood leakage occurred 61% of the time,4 risking potential exposure to bloodborne pathogens (BBP).
Multiple PIVC insertion attempts
An emergency department study suggests that first attempt insertion success ranges from 18–79%.6
Multiple insertion attempts increase the risk to clinicians of needlestick injury (NSI) and potential exposure to BBP, and undue pain and increased infection risk to the patient.6
When clinician safety and patient comfort and care are critical, BD Cathena™ Safety IV Catheter with BD Multiguard™ Technology**:
Eliminates the need for venous compression
during insertion, connections and disconnections, and blood draws, reducing the risk of blood exposure.**,7
during insertion, connections and disconnections, and blood draws, reducing the risk of blood exposure.**,7
Reduces blood exposure risk**
by stopping blood flow during conections and reconnections.^,7
Provides immediate visual confirmation
of vessel entry with BD Instaflash™ Needle Technology, improving opportunities for first attempt insertion success.*,8,9
Provides protection from needlestick injuries (NSIs)
with integrated passive safety system.
Offers an ergonomic design
that is easily adaptable to preferred insertion techniqes and provides enhanced control throughout the procedure.
Clinically demonstrated to reduce phlebitis
by up to 69% with BD Vialon™ Catheter Material, compared to an FEP catheter material.10-12
Supports longer IV dwell times,
with BD Vialon™ Catheter Material.††,13
Smooth, tapered catheter tip design
with smooth, tapered catheter tip design.**
The BD Cathena™ Safety IV Catheter with BD Multiguard™ Technology is designed to enhance the confidence, care and comfort of clinicians and patients.







Without wings product no. | With wings product no. | Size | Inner diameter | Outer diameter | Gravity flow rate | Maximum power injector flow rate for constrast media viscoslty <=11.8 cP(mPa⋅s) | Maximum power injector flow rate for constrast media viscoslty <=27.5 cP(mPa⋅s) |
| BD Cathena™ Safety IV Catheter with BD Multiguard™ Technology | |||||||
386860 | 386880 | 24 G x .075 in
| 0.53 mm | 0.71 mm | 20 mL/min | 3.5 mL/sec | 2.5 mL/sec |
386861 | 386881 | 22 G x 1.00 in
| 0.67 mm | 0.90 mm | 36 mL/min
| 6.0 mL/sec | 3.5 mL/sec |
- | 386882 | 22 G x 2.00 in
| 0.67 mm | 0.90 mm | 29 mL/min
| 6.0 mL/sec | 3.5 mL/sec |
386862 | 386883 | 20 G x 1.00 in
| 0.83 mm | 1.10 mm | 64 mL/min | 11.0 mL/sec | 7.0 mL/sec |
386863 | 386884 | 20 G x 1.25 in
| 0.83 mm | 1.10 mm | 60 mL/min
| 11.0 mL/sec | 7.0 mL/sec |
368864 | 386885 | 20 G x 2.00 in
| 0.83 mm | 1.10 mm | 52 mL/min
| 11.0 mL/sec | 7.0 mL/sec |
386865 | 386886 | 18 G x 1.25 in
| 0.98 mm | 1.31 mm | 94 mL/min
| 16.0 mL/sec | 10.0 mL/sec |
386866 | 386887 | 18 G x 2.00 in
| 0.98 mm | 1.31 mm | 87 mL/min
| 16.0 mL/sec | 10.0 mL/sec |
386867 | 386888 | 16 G x 1.25 in | 1.36 mm | 1.74 mm | 190 mL/min
| 27.0 mL/sec | 18.0 mL/sec |
386868 | 386889 | 16 G x 2.00 in
| 1.36 mm | 1.74 mm | 176 mL/min
| 27.0 mL/sec | 18.0 mL/sec |
| BD Cathena™ Safety IV Catheter without BD Multiguard™ Technology | |||||||
386859 | 386879 | 24 G x 0.75 in | 0.53 mm | 0.71 mm | 20 mL/min
| 3.5 mL/sec | 2.5 mL/sec |
386871 | 386892 | 22 G x 1.00 in
| 0.67 mm | 0.90 mm | 37 mL/min
| 6.0 mL/sec | 3.5 mL/sec |
- | 386893 | 22 G x 2.00 in | 0.67 mm | 0.90 mm | 29 mL/min
| 6.0 mL/sec | 3.5 mL/sec |
386872 | 386894 | 20 G x 1.00 in
| 0.83 mm | 1.10 mm | 65 mL/min
| 11.0 mL/sec | 7.0 mL/sec |
386873 | 386895 | 20 G x 1.25 in
| 0.83 mm | 1.10 mm | 62 mL/min | 11.0 mL/sec | 7.0 mL/sec |
386874 | 386896 | 20 G x 2.00 in
| 0.83 mm | 1.10 mm | 52 mL/min
| 11.0 mL/sec | 7.0 mL/sec |
386875 | 386897 | 18 G x 1.25 in
| 0.98 mm | 1.31 mm | 104 mL/min
| 16.0 mL/sec | 10.0 mL/sec |
386876 | 386898 | 18 G x 2.00 in | 0.98 mm | 1.31 mm | 90 mL/min
| 16.0 mL/sec | 10.0 mL/sec |
386869 | 386890 | 16 G x 1.25 in
| 1.36 mm | 1.74 mm | 212 mL/min
| 27.0 mL/sec | 18.0 mL/sec |
386870 | 386891 | 16 G x 2.00 in
| 1.36 mm | 1.74 mm | 200 mL/min
| 27.0 mL/sec | 18.0 mL/sec |
BD's collection of literature on industry and on our offerings gives you information you can use to continue striving for excellence.
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
BD promotes clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.
Yes. BD Cathena™ Safety IV Catheter is MR Conditional. Please refer to the Instructions for Use for the specified conditions for use.
Yes. BD Cathena™ Safety IV Catheter are acceptable for use with power injectors up to 325 psi (2,240 kPa).
Yes. BD Cathena™ Safety IV Catheters are available with and without wings.
BD Cathena™ Safety Peripheral IV Catheter (PIVC) with BD Multiguard™ Technology is a multi-use blood control catheter that does not require venous compression during insertion and subsequent luer connection and disconnection.^
BD Cathena™ Safety IV Catheters feature BD Vialon™ Catheter Material. BD Vialon™ is a polyurethane material that has been clinically demonstrated to reduce phlebitis up to 69% compared to an FEP catheter material.10,11,12,*
BD Cathena™ Safety IV Catheter features BD Instaflash™ Needle Technology designed to provide immediate visual confirmation of vessel entry (flashback) and was clinically demonstrated to improve first-attempt insertion success, reducing painful hit-and-miss insertions.8,9,*
No. BD Cathena™ Safety IV Catheter has a passive needle shield that automatically covers the needle as it is removed from the catheter, protecting against needlestick injuries and exposure to blood.
Yes. BD Cathena™ Safety IV Catheter can be used to draw blood. BD Cathena™ Catheters are intended to be inserted into a patient's peripheral vascular system for short-term use to sample blood, monitor blood pressure, or administer fluids.
Yes. The BD Cathena™ Safety IV Catheter is ergonomically designed to be easily adaptable to current insertion techniques, while the anti-rotation tab is designed to enhance tactile control during advancement.
Disclaimers:
*Compared to a non-notched needle
†Compared to an FEP catheter
^For up to 10 seconds
**Compared to a non-safety, non-blood control catheter
††Compared to PTFE catheter material
BD-42420 (05/23)