-
CapSure™ Permanent Fixation System - 30 Permanent Fasteners
SKU/REF 0113230
-
CapSure™ Permanent Fixation System - 15 Permanent Fasteners
SKU/REF 0113215
Canada
true

- Overview
- Products & Accessories
- EIFU & Resources

REDEFINED FASTENER DESIGN
316L stainless steel
- 316L stainless steel is a surgical stainless steel commonly used in biomedical implants that are put under pressure including bone screws and prostheses
Smooth PEEK cap
- Cap is made from polyetheretherketone (PEEK). PEEK is an inert organic thermoplastic polymer, considered an advanced biomaterial
PEEK is used in many medical implants including dental implants, heart valves and stents, and joint prostheses

DESIGNED FOR OPTIMIZED CLINICAL BENEFITS
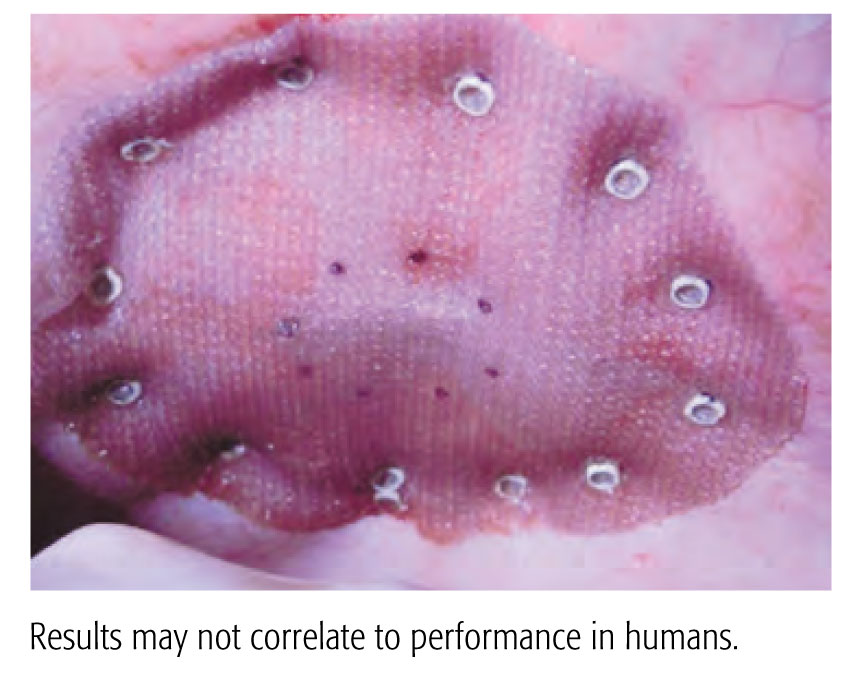
A 90 Day Preclinical Adhesion Study Demonstrated Stronger Results with the CapSure™ Fixation System vs. ProTack™ Fixation System.
Evaluation of a Novel Permanent Capped Helical Coil Fastener in a Porcine Model of Laparoscopic Ventral Hernia Repair
Arnab Majumder, Mojtaba Fayezizadeh, William W. Hope,
Yuri W. Novitsky • Surgical Endoscopy April 2016
- Significantly less adhesion coverage
- Greater percentage of properly engaged fasteners
- Greater mesh/tissue integration
- Shielding exposed fastener points on the visceral mesh surface with polymer caps is suggested by the data to reduce adhesion formation and aid in mesh fixation and integration
Preclinical data. Results may not correlate to performance in humans.
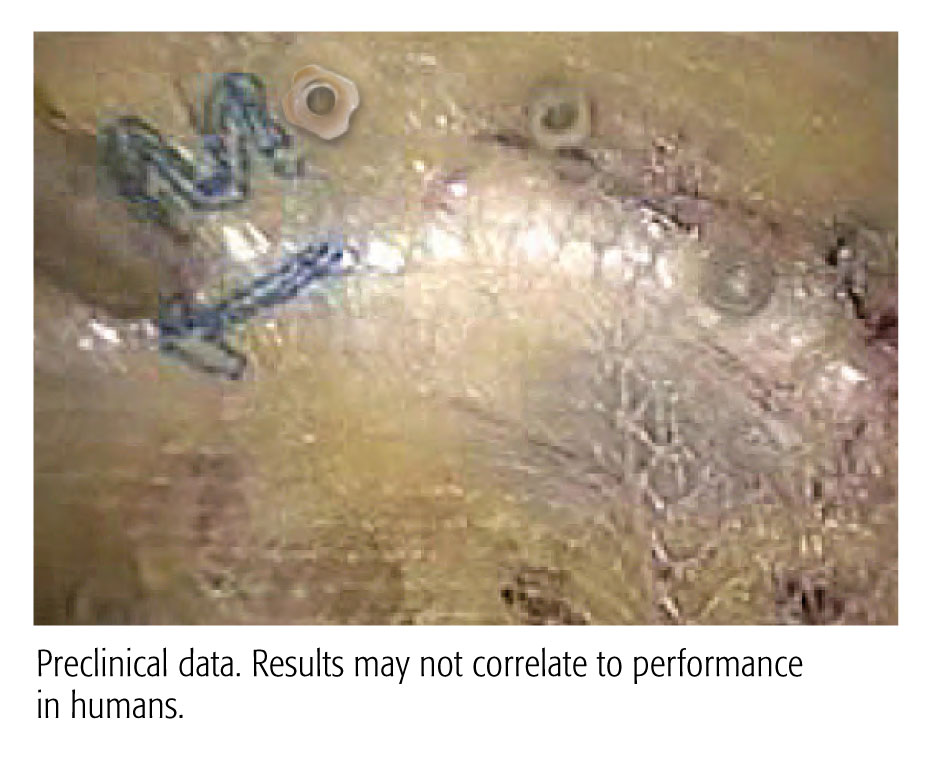
STRONG REPAIR
CapSure™ fastener easily penetrates Cooper’s ligament and underlyingstructures, equivalent to ProTack™
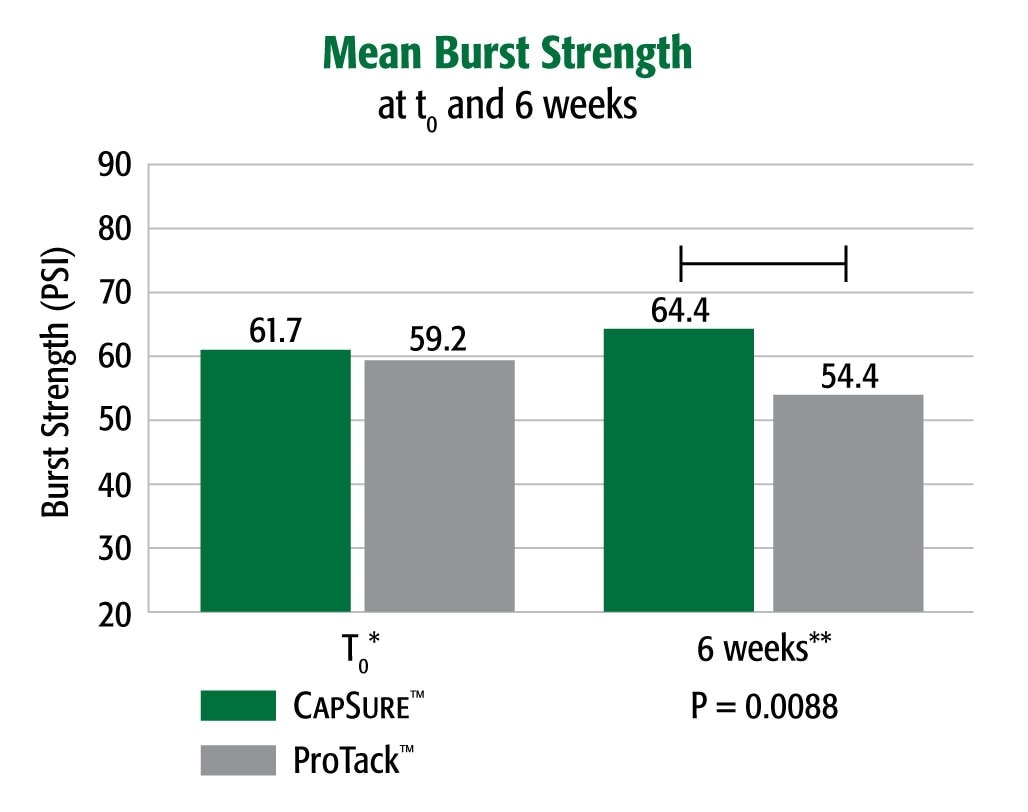
Equivalent Strength to ProTack™ at t0, Greater Strength Over Time
Burst strength testing demonstrated that in a porcine model CapSure™ fixated Ventralight™ ST Mesh had a greater (4.3%) burst strength at t0 and significantly higher (18.4%) peak burst strength at 6 weeks post-implantation than did ProTack™ fixated Ventralight™ ST Mesh at the same time point (p = .0088).
* Porcine abdominal wall tissue:
Animal data may not correlate to performance in humans
* Porcine 6 week implant study:
Animal data may not correlate to performance in humans
Reliable
Better confidence in your delivery system performance
- Rotary drive system with a comfortable handle and easy to deploy trigger, with an average delivery force that is 30% less than ProTack™1
- Consistent fastener deployment and depth of tissue purchase – preclinical studies demonstrated more favorable fastener seating results versus ProTack™2
- Available in 15 and 30 fastener count devices providing significant savings per device over ProTack™, for repairs where 15 or less fasteners are required
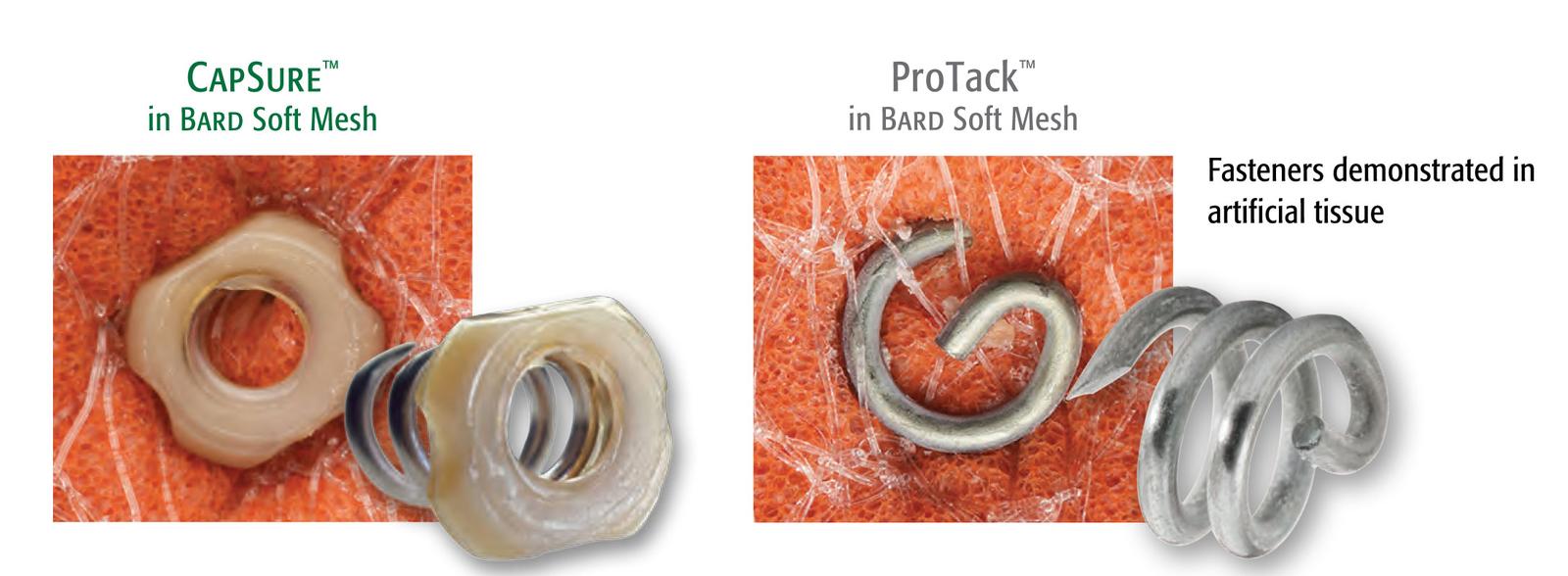
CapSure™ Fasteners
CapSure™ Fasteners have 2X the mesh surface area coverage to hold mesh in place ensuring secure fixation and more visible fasteners. Bench testing with 3DMax™ Light demonstrates that CapSure™ is 15X more likely to retain large pore mesh versus ProTack™1
Literature
BD's collection of literature on industry and on our offerings gives you information you can use to continue striving for excellence.
Learn more
Training
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
Learn more
Events
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
Learn more
Case Studies
BD promotes clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.
Learn more
true
