Canada


- Overview
- Clinical Experience
- EIFU & Resources
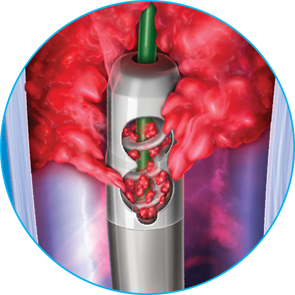
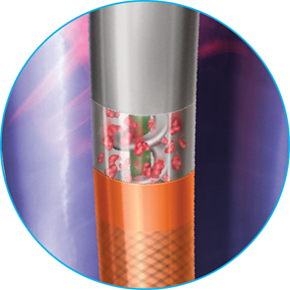
Dynamic 3-in-1 Mechanism of Action
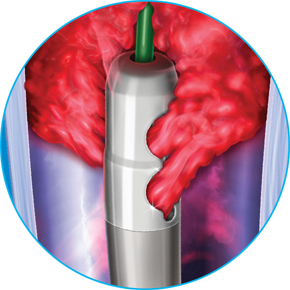
ADVANCED ASPIRATION
Internal helix rotates at 40,000 – 60,000 RPMs creating powerful suction at the catheter tip, aspirating clot through wall-apposed side windows
CONTINUOUS MACERATION
Clot is macerated by the internal helix as it enters the catheter, and is continuously macerated as it travels through the catheter to help mitigate clogging
MECHANICAL TRANSPORATION
Internal helix helps transport clot through the catheter, out of the vessel
Small Footprint & Simple Set-Up
- No Warm-up or repeated catheter clean-out required
- Plug-and-play capital component

- 1
- 2
- 3
- 4
- 5
- 6
Control Unit
Small, portable design; simple to set-up; plug-in and
switch on; connects to both hand and foot motor
Hand Motor
Operated by hand to facilitate single-operator scenarios; magnetic coupling facilitates ease of use while in the sterile environment
Ergonomic Handle
Easy to use handle designed for single operator control; disposable catheter simply clips to reusable portion of the handle
Aspirex™ Catheter
Non-rotating, rounded catheter tip is designed for optimal performance in the venous anatomy; no defined limitation on treatable lesion length
Guidewire
Nitinol core shaft with PTFE coating for catheter support; hydrophilic-coated with a flexible, angled tip to enable lesion crossing; gold-plated tungsten coil to enhance visualization under fluoroscopy
Foot Motor
Optional-use foot-switch to facilitate single- or multiple-operator scenarios
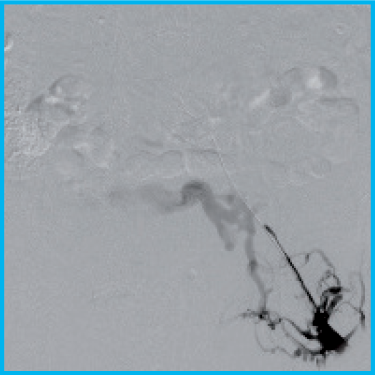
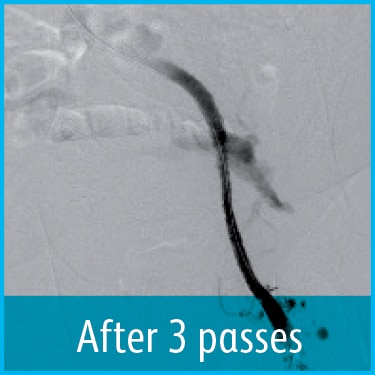
Recanalization of an Acute Iliofemoral Deep Vein Thrombosis Using the Aspirex™ 10F Catheter
Dr Michael Lichtenburg, Chief Medical Officer, Karolinen Hospital, Arnsberg, Germany
Venogram demonstrated complete thrombotic occlusion of the left iliac vein (figure 1). Mechanical Thrombectomy was performed with the 10F Aspirex™ Catheter (figure 2). At the 3 months clinical follow-up the patient presented symptom-free. Venous outflow was shown to be patent on the treated side.
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
*6F/8F catheters offer a single multi-sided L-shape window, 10F catheter offers two side windows.
The Aspirex™ Mechanical Aspiration Thrombectomy System is indicated for the removal of acute emboli and thrombi from vessels in the peripheral venous system.
The Aspirex™ Mechanical Aspiration Thrombectomy System is not for use in the vessels of the cardiac, pulmonary, coronary and neurovasculature. Please consult product labels and instructions for use for indications, contraindications, hazards, warnings, and precautions.
BD-56967