Canada
3DMax™ Light Mesh
Lighter-weight version of the popular 3DMax™ Mesh, featuring a large pore knit design.

- Overview
- Products & Accessories
- EIFU & Resources
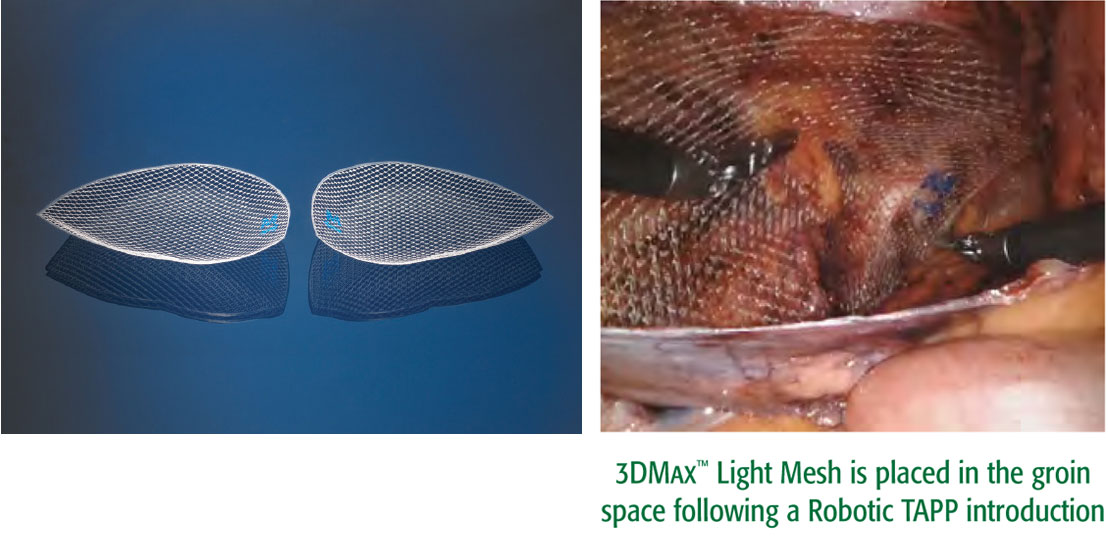
A lightweight 3D-shaped mesh for laparoscopic approaches such as TAPP, TEP and Robotic TAPP
This lighter-weight version of our popular 3DMax™ Mesh features a large pore knit. It is easy to deploy and provides excellent visibility. It is designed to conform to the inguinal anatomy and retains its shape following laparoscopic introduction, including a robotic approach.
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
Unique
- 3D shape developed by a laparoscopic surgeon.
- Designed to conform to the inguinal anatomy.
- Contour minimizes buckling that may be seen with flat mesh.
- Design may reduce the need for fixation.
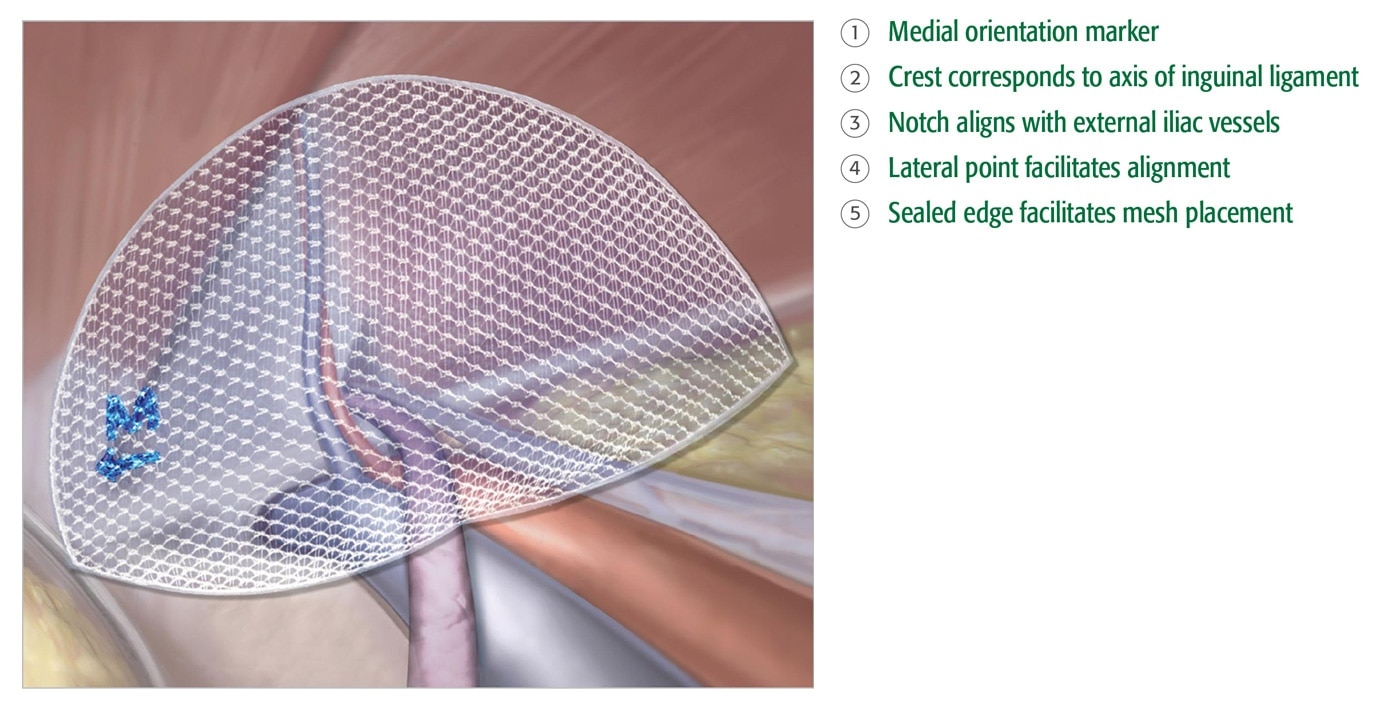
Precise
- Sealed edge and medial orientation marker facilitate accurate placement and positioning.
- Built-in memory maintains shape.
- 3DMax™ Light mesh is available in 3 sizes and in both left and right orientation.
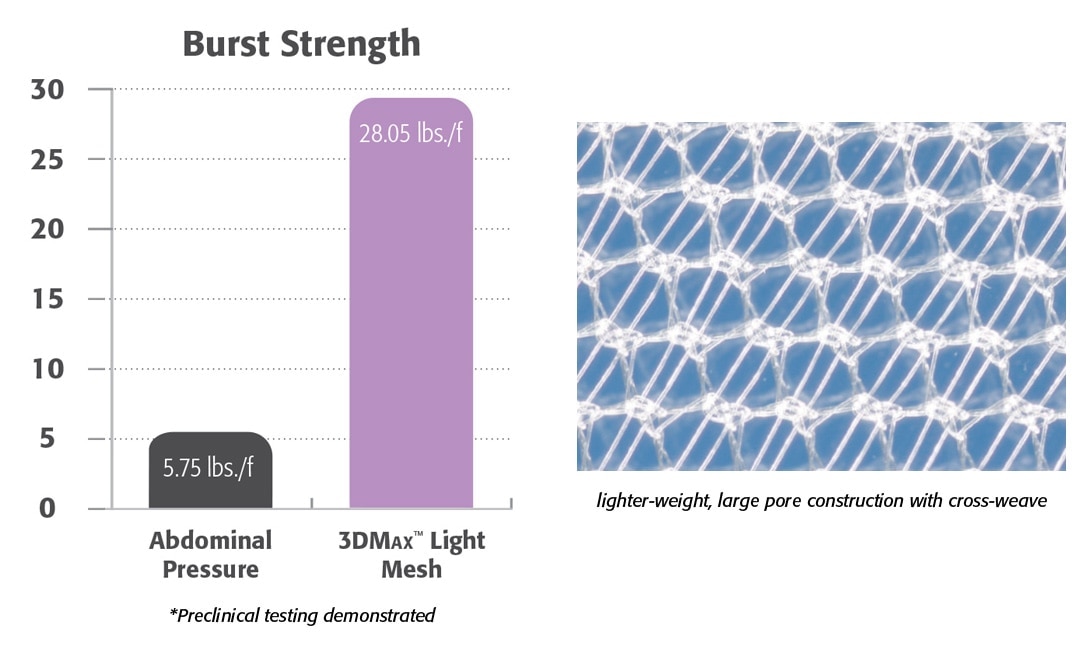
Lighter Weight
- Lighter-weight monofilament polypropylene mesh.
- Large pore knit provides excellent visibility.
- Preclinical data demonstrated the formation of a flexible and compliant abdominal wall.1
- TAPP
- TEP
- Robotic TAPP
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.


