RELATED PRODUCTS NOT AVAILABLE
true
Support
Singapore (Greater Asia Head Office)
+65 6664 2700
bd_sea@bd.com
Thank you for contacting our sales team!
A sales representive will get in touch with you shortly.
Address
2 International Business Park Road, #08-08 The Strategy, Singapore 609930


- Overview
- Products & Accessories
- EIFU & Resources
false
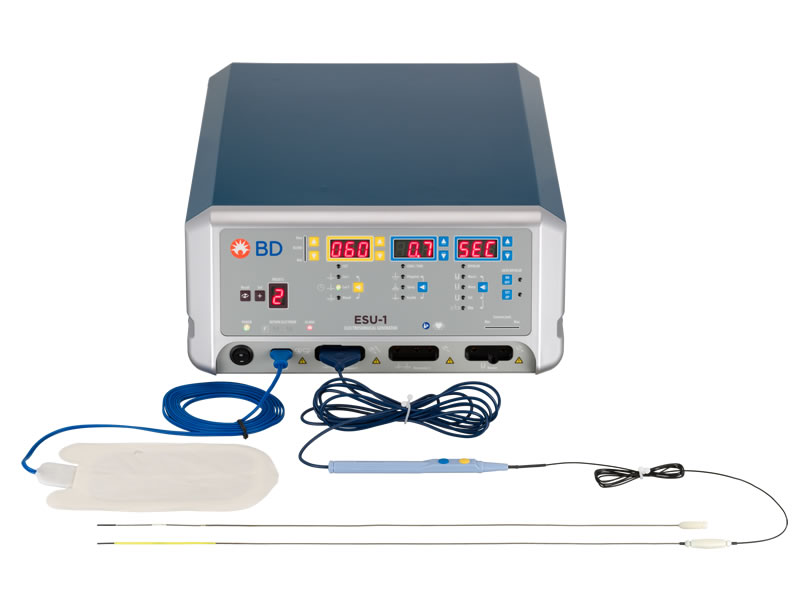
WavelinQ™ EndoAVF System
The WavelinQ™ EndoAVF System consists of two 4 French (4F) single-use, disposable, magnetic, hydrophilic coated catheters: a venous catheter and an arterial catheter. It is used with an Electrosurgical Unit (ESU) and Electrosurgical Pencil.

WavelinQ™ EndoAVF Arterial Catheter
The WavelinQ™ EndoAVF System arterial catheter contains a backstop for receiving the electrode. When placed in proximity, the magnets contained in each catheter attract, while aligning the electrode with the backstop. Two rotational indicators, present in each catheter, are used to accurately position the catheters. The catheters may be advanced and engaged in either a parallel (same direction) or antiparallel (opposite direction) configuration.

WavelinQ™ EndoAVF Venous Catheter
The WavelinQ™ EndoAVF System venous catheter is a flexible 4F magnetic catheter that contains a radiofrequency (RF) electrode for the delivery of RF energy, a yellow valve crosser on the distal end, and a cable and plug. The WavelinQ™ venous catheter connects via an Electrosurgical Pencil to an ESU for delivery of radiofrequency energy with standard grounding pads. RF energy can then be delivered through the electrode for cutting and/or coagulating tissue.
true
Literature
BD's collection of literature on industry and on our offerings gives you information you can use to continue striving for excellence.
Learn more
true
Please consult product labels and inserts for indications, contraindications, hazards, warnings, precautions and directions for use.
BD-23460
true