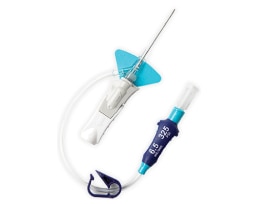
- Overview
- Products & Accessories
- EIFU & Resources
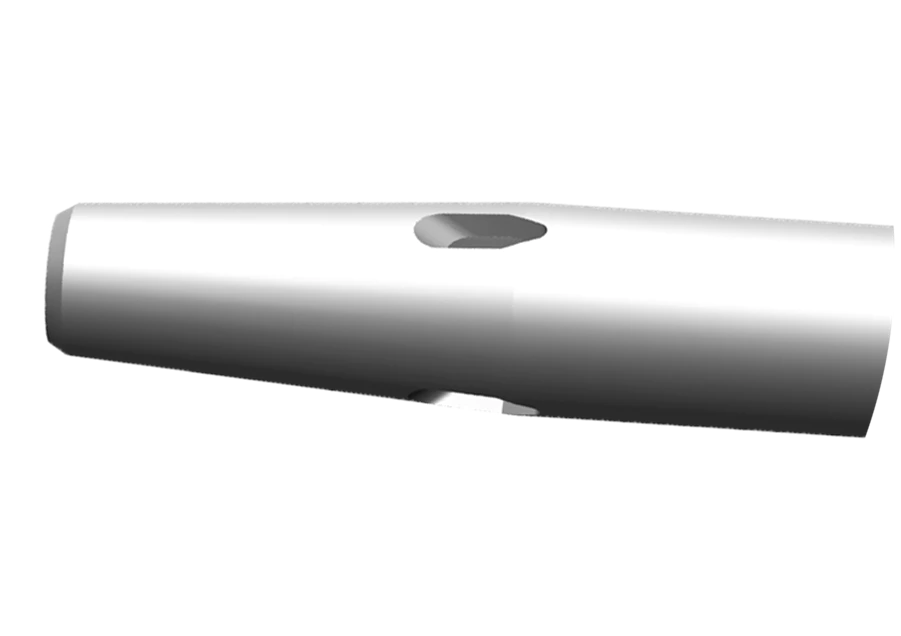
- Features multiple teardrop-shaped holes diffuse flow and reduce forces that can cause catheter motion in the vein by up to 67% during contrast enhanced CT procedure.
- Enables higher flow rates on smaller gauge catheters (22 to 24 G) for power injection.*
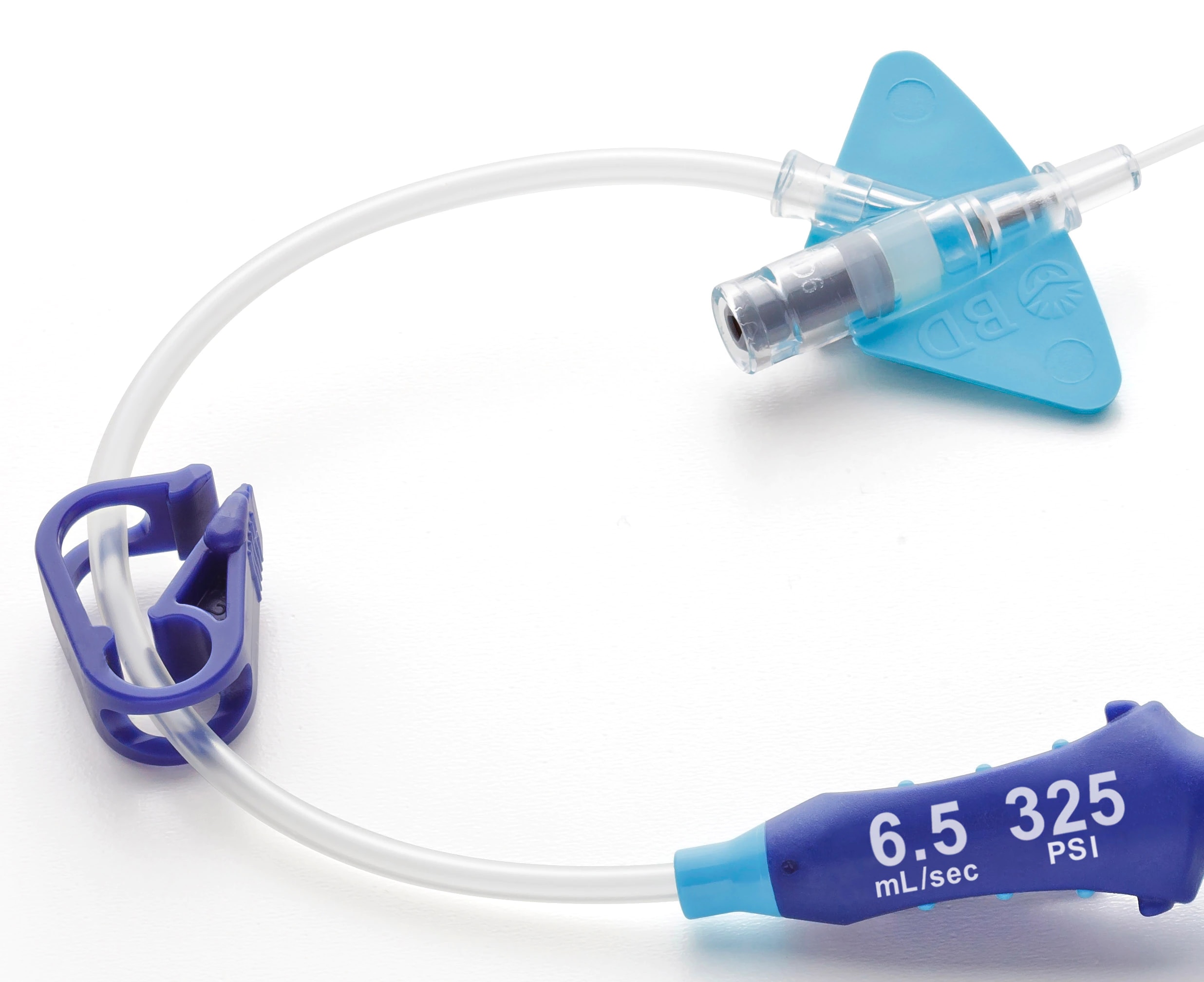
- The system features an all-in-one catheter and extension set built for your power injector's 325 psi setting.
- A unique all-in-one closed system designed to address common CT IV challenges during power injection procedures.*
- Needs fewer add-on devices, minimizing the number of manipulations which may lead to touch contamination and accidental disconnections.4,5 *
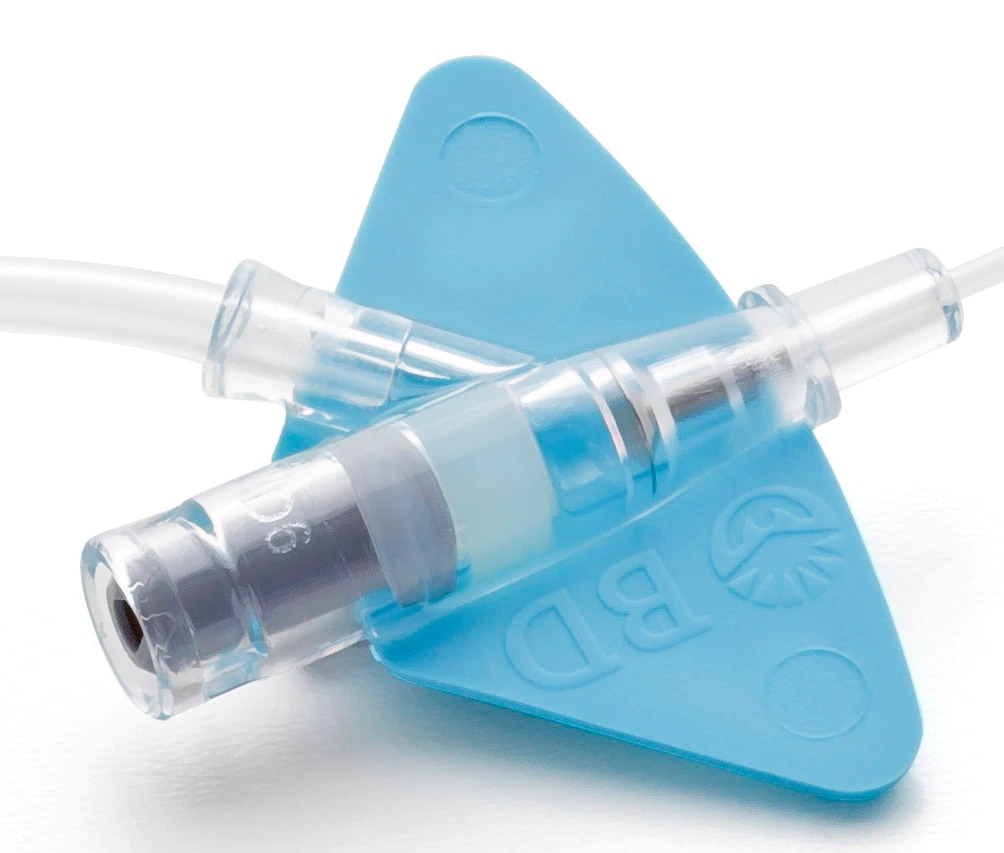
- The BD Nexiva Diffusics system incorporates a built-in stabilization platform.
- Minimizes movement and manipulation at the insertion site reducing accidental dislodgement.2**

- Designed to confirm immediate vessel entry.
- Quick blood visualization may help improve insertion success and therefore reduce insertion attempts.

- Clinically demonstrated to dwell up to 144 hours.1
- Reduces the chance of mechanical phlebitis by up to 50%.6†

- The system features a luer adapter with indication of maximum power injection flow rates and pressure setting.
Catheter stabilization is recognized as an intervention to decrease the risk for phlebitis, catheter migration and dislodgement and may be advantageous in preventing catheter-related bloodstream infections (CRBSIs).7
Recommend limiting the use of add-on devices to reduce the potential for contamination, additional manipulation, and disconnection.4
98% reduced blood exposure during insertion due to the BD Nexiva IV catheter preassembled system.3*
Clinically demonstrated to reduce accidental dislodgement,3‡ meeting Infusion Nursing Society standards4 and CDC guidelines7 for catheter stabilization.
In a clinical study, results demonstrated a significant reduction in the rate of phlebitis (grade 2 or higher), PIVC-related complications, and infiltration in the closed system versus the open system group.1
This is a modal window.
The Video Cloud video was not found.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
BD Nexiva™ Diffusics™ Product Overview Animation
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
BD Nexiva™ Diffusics™ Closed IV Catheter Insertion Techniques
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
BD Nexiva™ Diffusics™ - Diffusion Animation
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
Notes
* Compared to a nondiffusion tip IV catheter
† Compared to a fluorinated ethylene propylene (FEP) catheter
**Compared to B. Braun Introcan Safety® catheter with Bard Statlock® IV Ultra stabilization device.
References
- Gonzalez Lopez J, Arribi Vilela A, Fernandez Del Palacio E, et al. Indwell times, complications and costs of open vs closed safety peripheral intravenous catheters: a randomized study. J Hosp Infect. 2014;86(2):117-126.
- Tamura N, Ave S, Hagimoto K, et al. Unfavorable peripheral intravenous catheter replacements an be reduced using an integrated closed intravenous catheter system. J Vasc Access. 2014;15(4):257-263.)
- Bausone-Gazda D, Lefaiver CA, Walters SA. A randomized controlled trial to compare the complications of 2 peripheral intravenous catheter-stabilization systems. J Infus Nurs. 2010;33(6):371-384.)
- Infusion Nurses Society;. Infusion therapy standards of practice. J Infus Nurs. 2016:39(1S)S72. Alexander M, Corrigan A. Gorski L, et al. Infusion nursing: an evidence based approach. 3rd ed. St. Louis, MO: Saunders Elsevier; 2010:410.
- Alexander M, Corrigan A. Gorski L, et al. Infusion nursing: an evidence based approach. 3rd ed. St. Louis, MO: Saunders Elsevier; 2010:410.
- Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheter. Annals of Internal Medicine. 1991;114:845-854.
- O'Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. CDC. 2011:16.