RELATED PRODUCTS NOT AVAILABLE
true
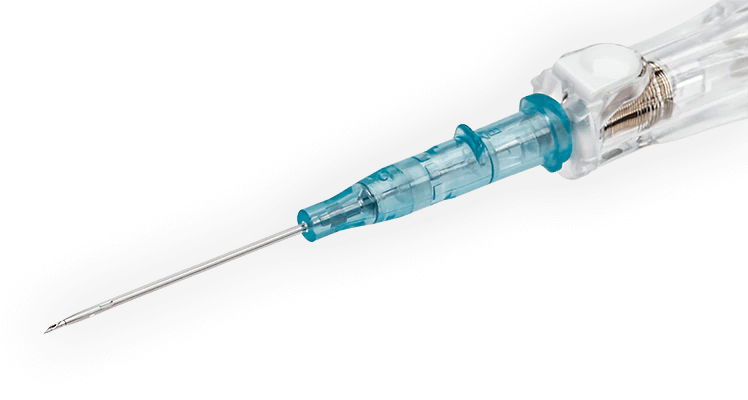
BD Insyte™ Autoguard™ BC Shielded IV Catheter with Blood Control Technology
Protect from blood leakage


- Overview
- Products & Accessories
- EIFU & Resources
The well-established BD Insyte™ Autoguard™ Shielded IV Catheter and BD Insyte-N™ Autoguard™ Shielded IV Catheter incorporates push button needle shielding technology that instantly retracts the needle, completely encasing it in the safety barrel, and helping reduce the risk of accidental needlestick injuries. It also features BD Instaflash™ Needle Technology for a greater likelihood of first attempt insertion success and clinically demonstrated BD Vialon™ Catheter Material.
Push-button Safety
Safety-engineered devices with automatic or semiautomatic (push-button needle shielding) are 10 times less likely to be associated with needlestick injuries.1*
First Attempt Insertion Success
BD Instaflash™ Needle Technology incorporates a notched needle, clinically demonstrated to significantly improve first-attempt insertion success, reducing painful hit-and-miss insertions2,3.
Longer Dwell Time and Lower Phlebitis
Proprietary BD Vialon™ Catheter Material softens, enabling longer dwell time and reducing the chance of phlebitis up to 69%4-6.
BD Insyte™ Autoguard™ BC product animation in-service video
Watch this video on vein penetration and blood control septum functionality with the BD Insyte Autoguard BC shielded IV catheter.
Literature
BD's collection of literature on industry and on our offerings gives you information you can use to continue striving for excellence.
Learn more
Training
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
Learn more
Events
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
Learn more
Case Studies
BD promotes clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.
Learn more
Notes
* Compared to a nonblood control IV catheter
† Compared to a nonsafety IV catheter
‡ Compared to a fluorinated ethylene propylene (FEP) catheter
References
- Onia R, Eshun-Wilson I, Arce C, et al. Evaluation of a new safety peripheral IV catheter designed to reduce mucocutaneous blood exposure. Curr Med Res Opin. 2011;27(7):1339-1346.
- Mendelson MH, Lin-Chen BY, Finkelstein-Blond L, et al. Evaluation of a safety IV catheter (IVC) (Becton Dickinson, Insyte Autoguard): final report (abstract). Presented at: Eleventh Annual Scientific Meeting Society for Healthcare Epidemiology of America; 2001.
*Compared to devices with fully manual safety features
1 Tosini W, Ciotti C, Goyer F, Lolom I, L'Hériteau F, Abiteboul D, Pellissier G, Bouvet E. Needlestick injury rates according to different types of safety-engineered devices: results of a French multicenter study. Infection Control & Hospital Epidemiology. 2010 Apr;31(4):402-7.
2van Loon FHJ, Timmerman R, den Brok GPH, Korsten EHM, Dierick-van Daele ATM, Bouwman ARA. The impact of a notched peripheral intravenous catheter on the first attempt success rate in hospitalized adults: block-randomized trial. JVA. 2021 DOI: 10.1177/1129729821990217.
3Seetharam AM, Raju U, Suresh K. A randomized controlled study to compare first stick success with Instaflash technology: The FIRSST study. JVA. 2022 DOI:10.1177/11297298221080369.
4Kus B, Buyukyilmaz F. Effectiveness of Vialon biomaterial versus Teflon catheters for peripheral intravenous placement: A randomized clinical trial. Jpn J Nurs Sci. 2020;e12328. https://doi.org/10.1111/jjns.12328
5Maki DG, Ringer M. Risk factors for infusion-related phlebitis with small peripheral venous catheters. Annals of Internal Medicine. 1991;114:845-854
6Gaukroger PB, Roberts JG, Manners TA. Infusion thrombophlebitis: a prospective comparison of 645 Vialon and Teflon cannulae in anaesthetic and postoperative use. Anaesthesia and Intensive Care. 1988;16:265-271
true