- AH is an absorbable powdered hemostatic agent intended for application to surgical wound sites to control bleeding. It is a fine, dry, sterilized white powder that is biocompatible, non-pyrogenic, and typically absorbed within 24-48 hours."

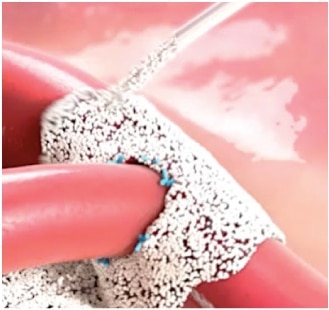
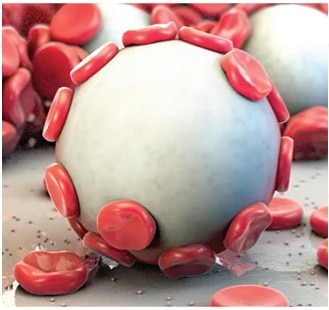
Arista™ AH is a 100% plant based absorbable surgical hemostatic powder derived from purified plant starch. The power of Arista™ AH lies in its Microporous Polysaccharide Hemospheres, a patented blood clotting technology.
Arista™ AH is indicated in surgical procedures (except neurologic and ophthalmic) as an adjunctive hemostatic device to assist when control of capillary, venous, and arteriolar bleeding by pressure, ligature, and other conventional procedures are ineffective or impractical.
* Because there have been reports of decreased amylase activity in newborns up to 10 months, absorption rates of Arista™ AH in this population may be longer than 48 hours.
Proprietary MPH™ Technology: A Unique Approach to Achieving Hemostasis
The power of Arista™ AH lies in its proprietary MPH™ (Microporous Polysaccharide Hemospheres) technology. Consisting of microporous particles with a controlled pore size, the spheres are designed to act as a molecular sieve. The powerful osmotic action dehydrates and gels the blood on contact to accelerate the natural clotting process.

1 Arista™ AH PMA P050038 Clinical Study.
2 See full Instructions for Use for detailed application instructions.
3 Arista™ AH Instructions for Use.
INDICATIONS
Arista™ AH is indicated in surgical procedures (except neurological and ophthalmic) as an adjunctive hemostatic device to assist when control of capillary, venous, and arteriolar bleeding by pressure, ligature, and other conventional procedures are ineffective or impractical.
CONTRAINDICATIONS
Do not inject or place Arista™ AH into blood vessels as potential for embolization and death may exist.
WARNINGS
Arista™ AH is not intended as a substitute for meticulous surgical technique and the proper application of ligatures or other conventional procedures for hemostasis.
Once hemostasis is achieved, excess Arista™ AH should be removed from the site of application by irrigation and aspiration particularly when used in and around foramina of bone, areas of bony confine, the spinal cord, and/or the optic nerve and chiasm. Arista™ AH swells to its maximum volume immediately upon contact with blood or other fluids. Dry, white Arista™ AH should be removed. The possibility of the product interfering with normal function and/or causing compression necrosis of surrounding tissues due to swelling is reduced by removal of excess dry material.
Safety and effectiveness of Arista™ AH have not been clinically evaluated in children and pregnant women. Because there have been reports of decreased amylase activity in newborns up to 10 months, absorption rates of Arista™ AH in this population may be longer than 48 hours.
Arista™ AH should be used with caution in the presence of infection or in contaminated areas of the body. If signs of infection or abscess develop where Arista™ AH has been applied, re-operation may be necessary in order to allow drainage.
Safety and effectiveness in neurosurgical and ophthalmic procedures has not been established.
Arista™ AH should not be used for controlling post-partum bleeding or menorrhagia.
PRECAUTIONS
When Arista™ AH is used in conjunction with autologous blood salvage circuits, carefully follow instructions in the Administration section of the IFU regarding proper filtration and cell washing.
Arista™ AH is intended to be used in a dry state. Contact with saline or antibiotic solutions prior to achieving hemostasis will result in loss of hemostatic potential.
Arista™ AH is not recommended for the primary treatment of coagulation disorders.
No testing has been performed on the use of Arista™ AH on bone surfaces to which prosthetic materials are to be attached with adhesives and is therefore not recommended.
Arista™ AH is supplied as a sterile product and cannot be resterilized. Unused, open containers of Arista™ AH should be discarded.
Do not apply more than 50g of Arista™ AH in diabetic patients as it has been calculated that amounts in excess of 50g could affect the glucose load.
In urological procedures, Arista™ AH should not be left in the renal pelvis or ureters to eliminate the potential foci for calculus formation.
ADVERSE REACTIONS
None of the adverse events that occurred in a randomized prospective, concurrently controlled clinical trial were judged by the Data Safety Monitoring Board to be related to the use of Arista™ AH. The most common recorded adverse events were pain related to surgery, anemia, nausea, lab values out of normal range, arrhythmia, constipation, respiratory dysfunction and hypotension – all reported in greater than 10% of the Arista™ AH treated patients. The details of this clinical trial’s adverse events can be reviewed in the IFU supplied with the product and are also available at www.bd.com.
Caution: Federal (USA) law restricts this device to sale by or on order of a licensed physician or properly licensed practitioner.
BD-14602
What is an Absorbable Hemostat?
Absorbable hemostatic agents are useful as adjunctive therapy during surgical procedures when conventional methods do not control surgical bleeding. 1

Hemostatic agents are substances to help prevent/stop bleeding from the bleeding site and involves the dependent reactions of plasma proteins, calcium ions and blood platelets which softens the platelet plug from the fibrinogen-to-fibrin conversion.
1 Arista™ AH PMA P050038 Clinical Study.
2 See full Instructions for Use for detailed application instructions.
3 Arista™ AH Instructions for Use.
INDICATIONS
Arista™ AH is indicated in surgical procedures (except neurological and ophthalmic) as an adjunctive hemostatic device to assist when control of capillary, venous, and arteriolar bleeding by pressure, ligature, and other conventional procedures are ineffective or impractical.
CONTRAINDICATIONS
Do not inject or place Arista™ AH into blood vessels as potential for embolization and death may exist.
WARNINGS
Arista™ AH is not intended as a substitute for meticulous surgical technique and the proper application of ligatures or other conventional procedures for hemostasis.
Once hemostasis is achieved, excess Arista™ AH should be removed from the site of application by irrigation and aspiration particularly when used in and around foramina of bone, areas of bony confine, the spinal cord, and/or the optic nerve and chiasm. Arista™ AH swells to its maximum volume immediately upon contact with blood or other fluids. Dry, white Arista™ AH should be removed. The possibility of the product interfering with normal function and/or causing compression necrosis of surrounding tissues due to swelling is reduced by removal of excess dry material.
Safety and effectiveness of Arista™ AH have not been clinically evaluated in children and pregnant women. Because there have been reports of decreased amylase activity in newborns up to 10 months, absorption rates of Arista™ AH in this population may be longer than 48 hours.
Arista™ AH should be used with caution in the presence of infection or in contaminated areas of the body. If signs of infection or abscess develop where Arista™ AH has been applied, re-operation may be necessary in order to allow drainage.
Safety and effectiveness in neurosurgical and ophthalmic procedures has not been established.
Arista™ AH should not be used for controlling post-partum bleeding or menorrhagia.
PRECAUTIONS
When Arista™ AH is used in conjunction with autologous blood salvage circuits, carefully follow instructions in the Administration section of the IFU regarding proper filtration and cell washing.
Arista™ AH is intended to be used in a dry state. Contact with saline or antibiotic solutions prior to achieving hemostasis will result in loss of hemostatic potential.
Arista™ AH is not recommended for the primary treatment of coagulation disorders.
No testing has been performed on the use of Arista™ AH on bone surfaces to which prosthetic materials are to be attached with adhesives and is therefore not recommended.
Arista™ AH is supplied as a sterile product and cannot be resterilized. Unused, open containers of Arista™ AH should be discarded.
Do not apply more than 50g of Arista™ AH in diabetic patients as it has been calculated that amounts in excess of 50g could affect the glucose load.
In urological procedures, Arista™ AH should not be left in the renal pelvis or ureters to eliminate the potential foci for calculus formation.
ADVERSE REACTIONS
None of the adverse events that occurred in a randomized prospective, concurrently controlled clinical trial were judged by the Data Safety Monitoring Board to be related to the use of Arista™ AH. The most common recorded adverse events were pain related to surgery, anemia, nausea, lab values out of normal range, arrhythmia, constipation, respiratory dysfunction and hypotension – all reported in greater than 10% of the Arista™ AH treated patients. The details of this clinical trial’s adverse events can be reviewed in the IFU supplied with the product and are also available at www.bd.com.
Caution: Federal (USA) law restricts this device to sale by or on order of a licensed physician or properly licensed practitioner.
BD-14602
BD offers training resources to help improve your clinical practices as part of our goal of advancing the world of health.
BD supports the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
Examples of surgeries that Arista™ AH can be used in include:
PMA clinical trial (General, Orthopedic, Cardiac)
Cardiac & Vascular:
ENT:
General:
Urology
BD-53859